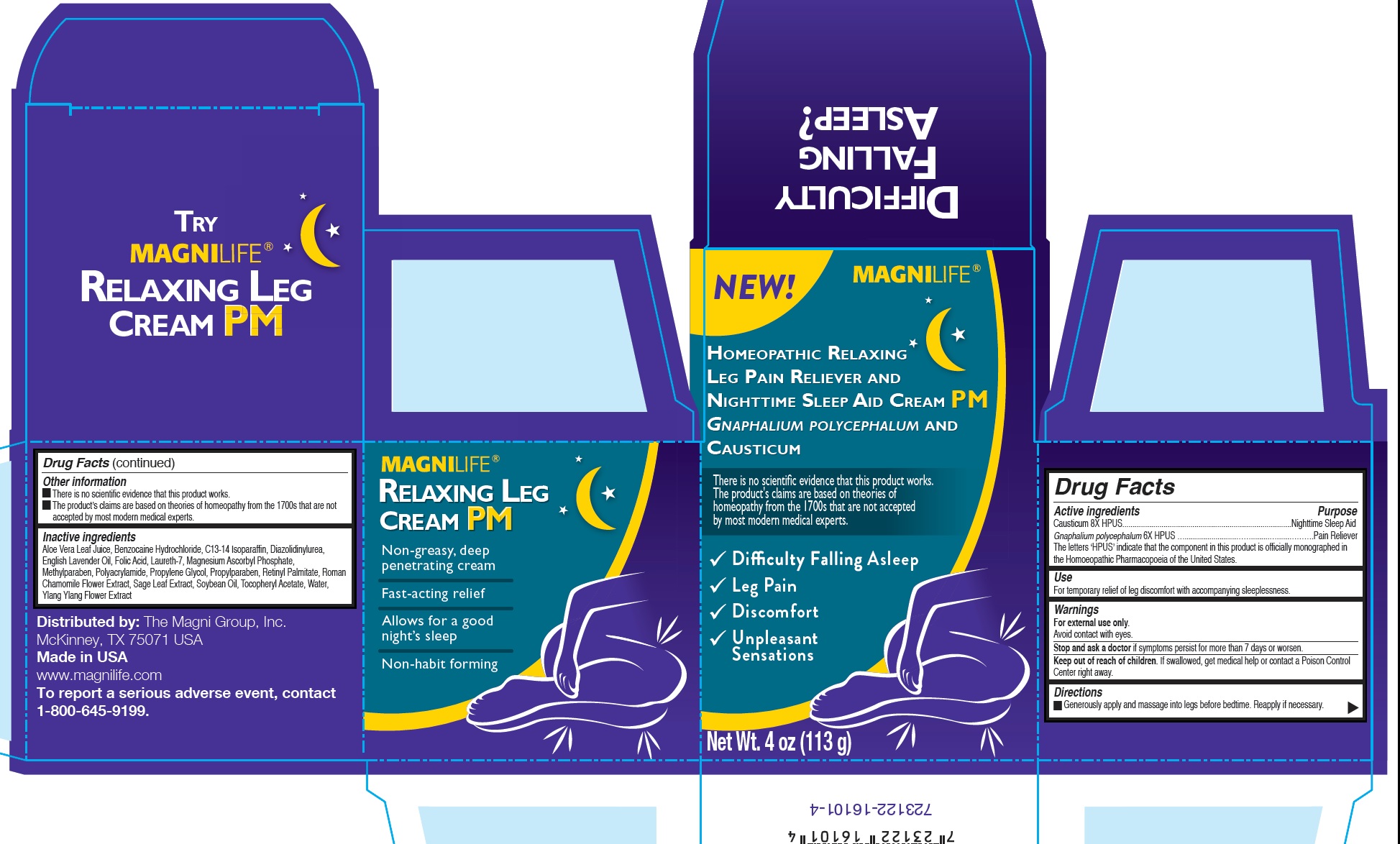
Label: RELAXING LEG CREAM HOMEOPATHIC PM- causticum, pseudognaphalium obtusifolium cream
- NDC Code(s): 43689-0027-1, 43689-0027-2
- Packager: The Magni Group Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aloe Vera Leaf Juice, Benzocaine Hydrochloride, C13-14 Isoparaffin, Diazolidinylurea, English Lavender Oil, Folic Acid, Laureth-7, Magnesium Ascorbyl Phosphate, Methylparaben, Polyacrylamide, Propylene Glycol, Propylparaben, Retinyl Palmitate, Roman Chamomile Flower Extract, Sage Leaf Extract, Soybean Oil, Tocopheryl Acetate, Water, Ylang Ylang Flower Extract
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
RELAXING LEG CREAM HOMEOPATHIC PM
causticum, pseudognaphalium obtusifolium creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43689-0027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 8 [hp_X] in 113 g PSEUDOGNAPHALIUM OBTUSIFOLIUM (UNII: 36XQ854NWW) (PSEUDOGNAPHALIUM OBTUSIFOLIUM - UNII:36XQ854NWW) PSEUDOGNAPHALIUM OBTUSIFOLIUM 6 [hp_X] in 113 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) CANANGA ODORATA FLOWER (UNII: 76GTF6Z97M) ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZOCAINE HYDROCHLORIDE (UNII: OG625Z9LEO) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) LAVENDER OIL (UNII: ZBP1YXW0H8) FOLIC ACID (UNII: 935E97BOY8) LAURETH-7 (UNII: Z95S6G8201) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SAGE (UNII: 065C5D077J) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43689-0027-1 113 g in 1 JAR; Type 0: Not a Combination Product 05/24/2017 2 NDC:43689-0027-2 1 in 1 JAR 05/24/2017 2 113 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/24/2017 Labeler - The Magni Group Inc (113501902)