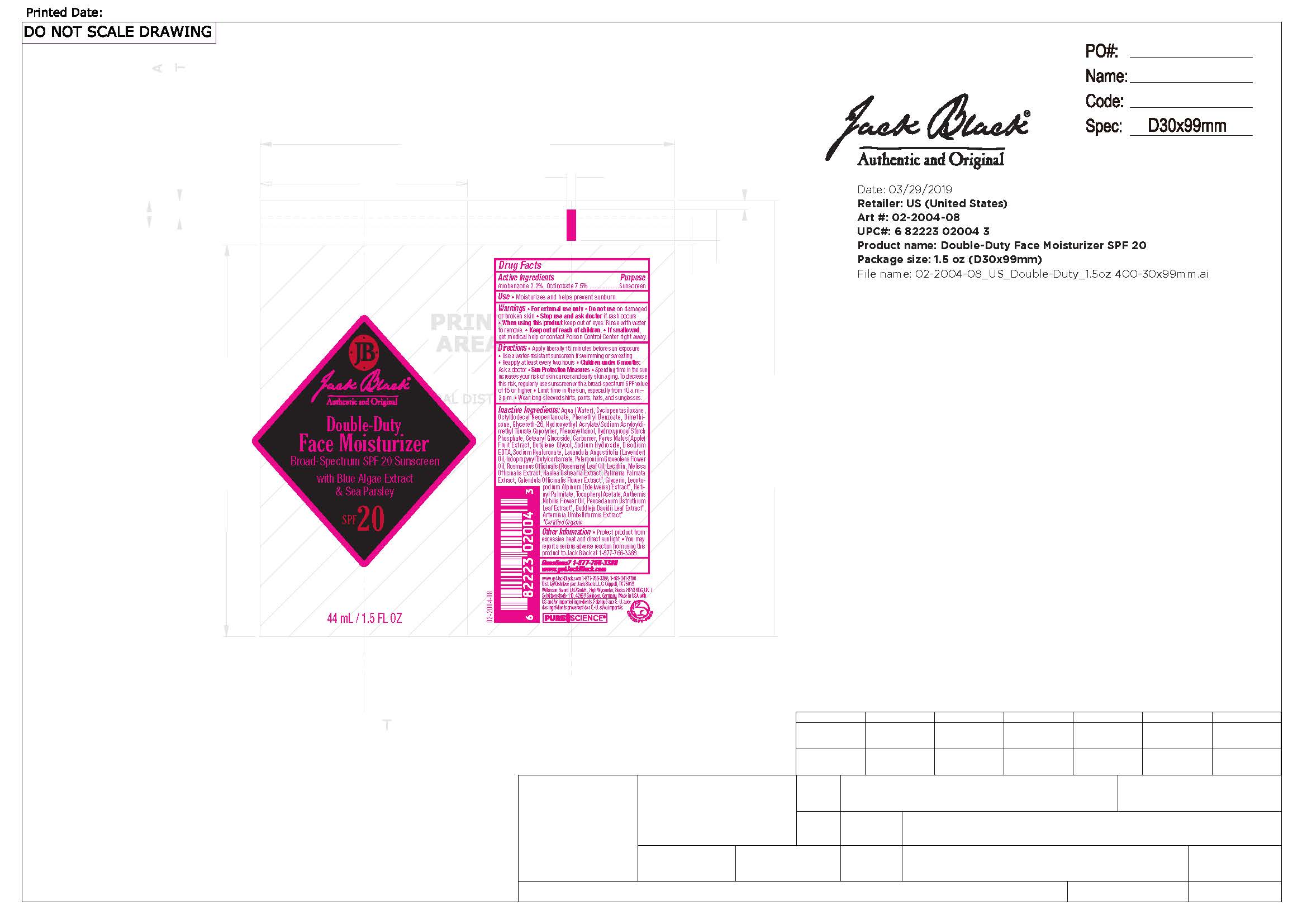
Label: JACK BLACK THE TRIPLE PLAY- avobenzone, octinoxate lotion
- NDC Code(s): 66738-661-40
- Packager: Jack Black L.L.C
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Use
- Warnings
- DO NOT USE
- STOP USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Apply liberally after cleansing and shaving and 15 minutes before sun exposure. • Use a water-resistant sunscreen if swimming or sweating. • Reapply at least every two hours. • Children under 6 months: Ask a doctor. Sun Protection Measures • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, use sunscreen with a broad spectrum SPF 15 or higher. • Limit time in the sun, especially from 10 a.m. – 2 p.m. • Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Water, Cyclopentasiloxane, Octyldodecyl Neopentanoate, Phenethyl Benzoate, Dimethicone, Glycereth-26, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Phenoxyethanol, Hydroxypropyl Starch Phosphate, Cetearyl Glucoside, Carbomer, Pyrus Malus (Apple) Fruit Extract, Butylene Glycol, Sodium Hydroxide, Disodium EDTA, Sodium Hyaluronate, Lavandula Angustifolia (Lavender) Oil, Iodopropynyl Butylcarbamate, Pelargonium Graveolens Flower Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Lecithin, Melissa Officinalis Extract, Haslea Ostrearia Extract, Palmaria Palmata Extract, Calendula Officinalis Flower Extract*, Glycerin, Leontopodium Alpinum (Edelweiss) Extract*, Retinyl Palmitate, Tocopheryl Acetate, Anthemis Nobilis Flower Oil, Peucedanum Ostruthium Leaf Extract*, Buddleja Davidii Leaf Extract*, Artemisia Umbelliformis Extract*.
* Certified Organic
- Other information
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JACK BLACK THE TRIPLE PLAY
avobenzone, octinoxate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66738-661 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.2 g in 100 mL Inactive Ingredients Ingredient Name Strength OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CORN OIL (UNII: 8470G57WFM) PHENETHYL BENZOATE (UNII: 0C143929GK) PALMARIA PALMATA (UNII: 7832HOY4ZQ) GLYCERIN (UNII: PDC6A3C0OX) ROSEMARY OIL (UNII: 8LGU7VM393) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) PHENOXYETHANOL (UNII: HIE492ZZ3T) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CHAMAEMELUM NOBILE FLOWER OIL (UNII: UB27587839) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HASLEA OSTREARIA (UNII: Y87200QHN9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERETH-26 (UNII: NNE56F2N14) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALCOHOL (UNII: 3K9958V90M) DIMETHICONE (UNII: 92RU3N3Y1O) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) MELISSA OFFICINALIS (UNII: YF70189L0N) LEONTOPODIUM ALPINUM FLOWERING TOP (UNII: QQC1AK06RK) BUDDLEJA DAVIDII LEAF (UNII: X380815D32) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) APPLE (UNII: B423VGH5S9) ARTEMISIA UMBELLIFORMIS FLOWER (UNII: 91OLL9AJ7D) PHOSPHATIDYLCHOLINE, SOYBEAN (UNII: 1T6N4D9YV6) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PEUCEDANUM OSTRUTHIUM LEAF (UNII: 86P27YRR6Y) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) HYDROXYPROPYL DISTARCH PHOSPHATE, HIGH AMYLOSE CORN (UNII: 9F8ET54T05) LAVENDER OIL (UNII: ZBP1YXW0H8) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66738-661-40 44 mL in 1 KIT; Type 1: Convenience Kit of Co-Package 09/30/2020 06/01/2024 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/30/2020 06/01/2024 Labeler - Jack Black L.L.C (847024036) Establishment Name Address ID/FEI Business Operations Swiss American CDMO 080170933 manufacture(66738-661)