Label: MICARDIS HCT- telmisartan and hydrochlorothiazide tablet
- NDC Code(s): 0597-0042-37, 0597-0043-37, 0597-0044-37
- Packager: Boehringer Ingelheim Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MICARDIS HCT safely and effectively. See full prescribing information for MICARDIS HCT. MICARDIS® HCT (telmisartan and ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue MICARDIS HCT as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
-
1 INDICATIONS AND USAGEMICARDIS HCT (telmisartan and hydrochlorothiazide) is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Initiate a patient whose blood pressure is not adequately controlled with telmisartan monotherapy 80 mg on MICARDIS HCT, 80 mg/12.5 mg orally once daily. Dose can be ...
-
3 DOSAGE FORMS AND STRENGTHS40 mg/12.5 mg, red and white tablets marked with the Boehringer Ingelheim logo and "H4" 80 mg/12.5 mg, red and white tablets marked with the Boehringer Ingelheim logo and "H8" 80 mg/25 mg, yellow ...
-
4 CONTRAINDICATIONSMICARDIS HCT is contraindicated: In patients who are hypersensitive to any component of this product [see Warnings and Precautions (5.5)]. In patients with anuria. For co-administration with ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Telmisartan - Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in labeling: Hypotension [see Warnings and Precautions (5.2)] Renal Impairment [see Warnings and Precautions (5.3)] Electrolytes and ...
-
7 DRUG INTERACTIONS7.1 Agents Increasing Serum Potassium - Co-administration of telmisartan with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - MICARDIS HCT can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
10 OVERDOSAGETelmisartan - Limited data are available with regard to overdosage of telmisartan in humans. The most likely manifestations of overdosage with telmisartan are hypotension, dizziness, and ...
-
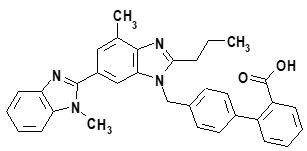
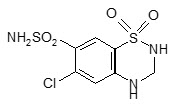
11 DESCRIPTIONMICARDIS HCT tablets are a combination of telmisartan, an orally active angiotensin II antagonist acting on the AT1 receptor subtype, and hydrochlorothiazide, a thiazide diuretic. Telmisartan, a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - MICARDIS HCT - MICARDIS HCT is a combination of two drugs with antihypertensive properties: a thiazide diuretic, hydrochlorothiazide, and an angiotensin II receptor ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Telmisartan and Hydrochlorothiazide - No carcinogenicity, mutagenicity, or fertility studies have been conducted with the ...
-
14 CLINICAL STUDIESTelmisartan and Hydrochlorothiazide - In controlled clinical trials with more than 2500 hypertensive patients, 1017 patients were exposed to telmisartan (20 mg to 160 mg) and concomitant ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMICARDIS HCT is available in three strengths as biconvex two-layered, oblong-shaped, uncoated tablets containing telmisartan and hydrochlorothiazide: 40 mg/12.5 mg tablet: red and white (may ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy - Advise female patients of childbearing age about the consequences of exposure to MICARDIS HCT ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA - Licensed from: Boehringer Ingelheim International GmbH - Ingelheim, Germany - Copyright © 2022 Boehringer Ingelheim ...
-
Patient InformationMICARDIS® HCT (my-CAR-dis HCT)(telmisartan and hydrochlorothiazide tablets)Read this Patient Information before you start taking MICARDIS HCT tablets and each time you get a refill. There may be new information. This information does not take the place of talking to ...
-
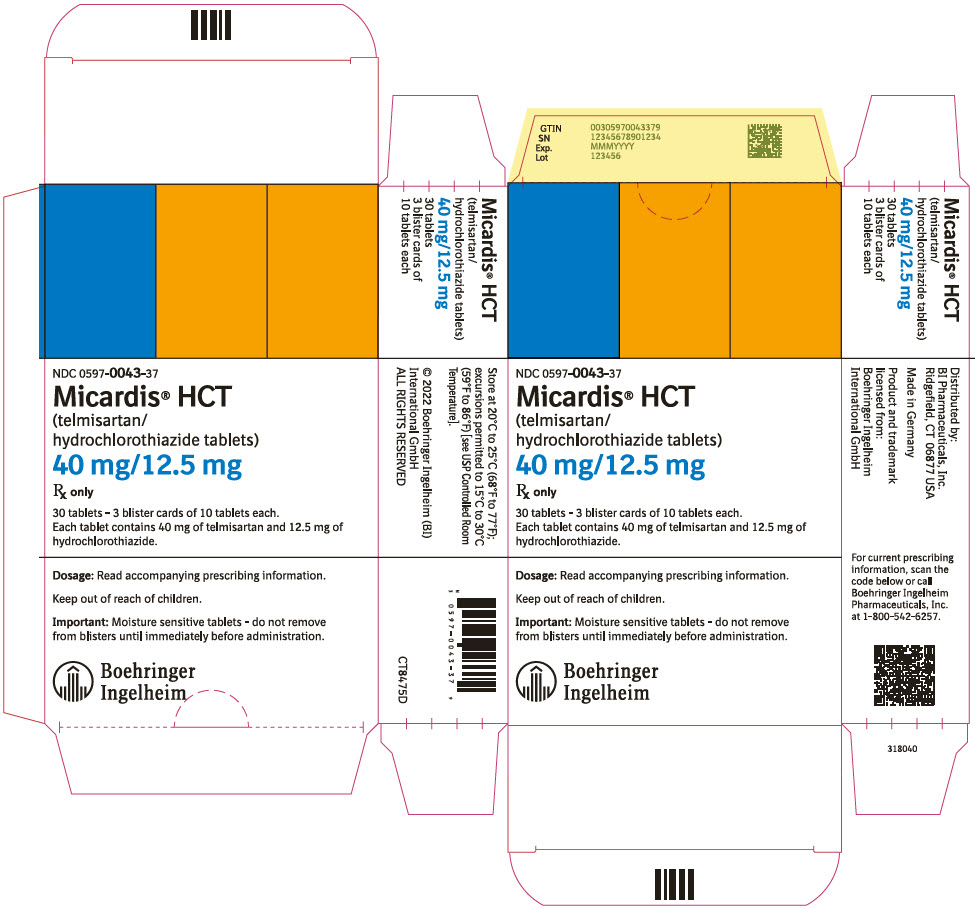
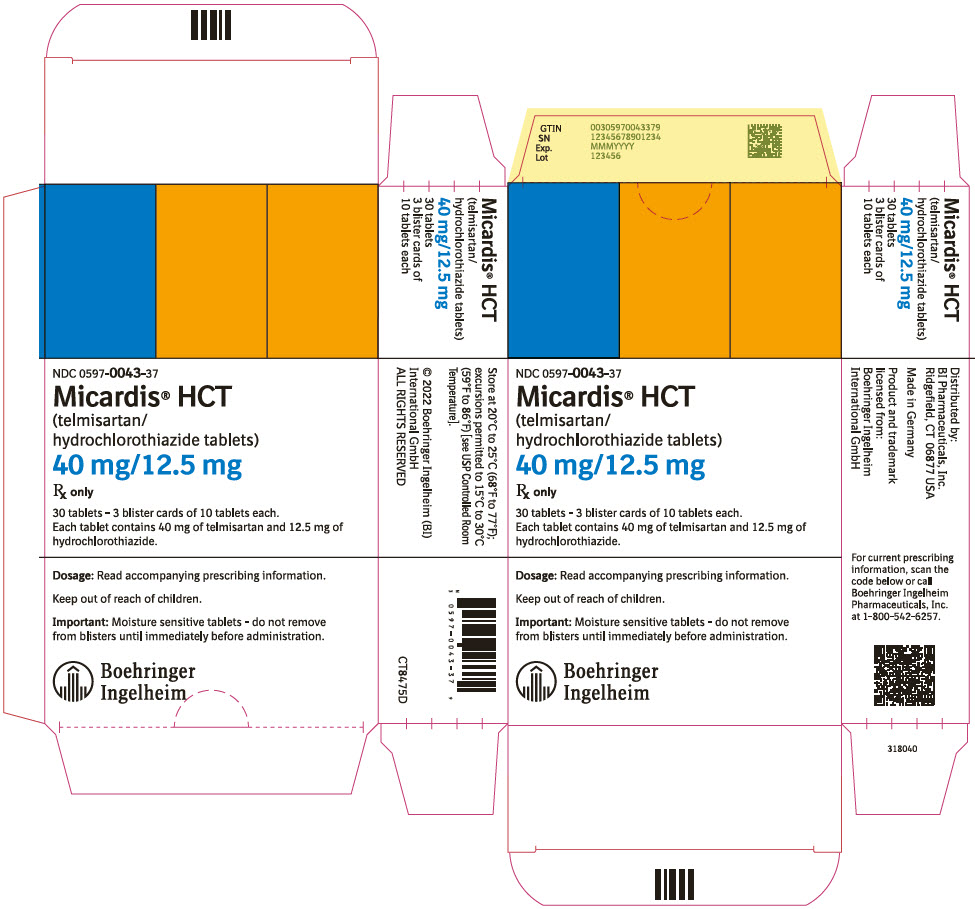
PRINCIPAL DISPLAY PANEL - 40 mg/12.5 mg Tablet Blister Card CartonNDC 0597-0043-37 - Micardis® HCT - (telmisartan/ hydrochlorothiazide tablets) 40 mg/12.5 mg - Rx only - 30 tablets - 3 blister cards of 10 tablets each. Each tablet contains 40 mg of telmisartan and ...
-
PRINCIPAL DISPLAY PANEL - 80 mg/12.5 mg Tablet Blister Card CartonNDC 0597-0044-37 - Micardis® HCT - (telmisartan/ hydrochlorothiazide tablets) 80 mg/12.5 mg - Rx only - 30 tablets - 3 blister cards of 10 tablets each. Each tablet contains 80 mg of telmisartan and ...
-
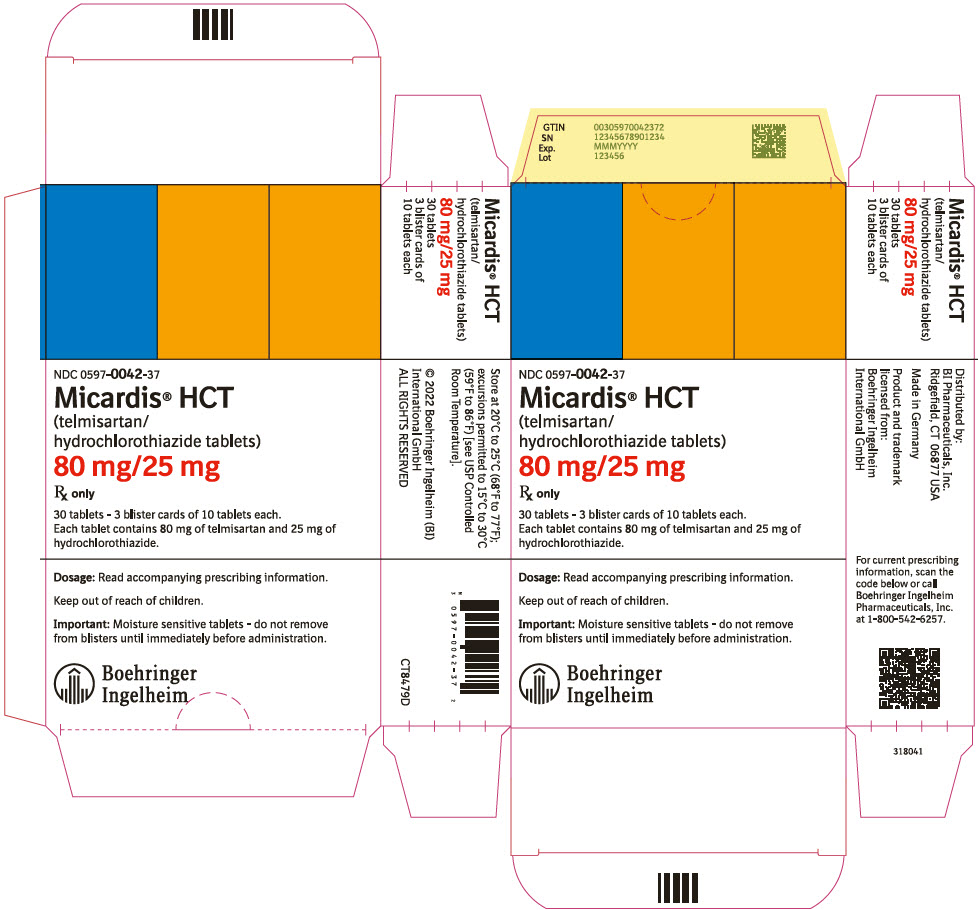
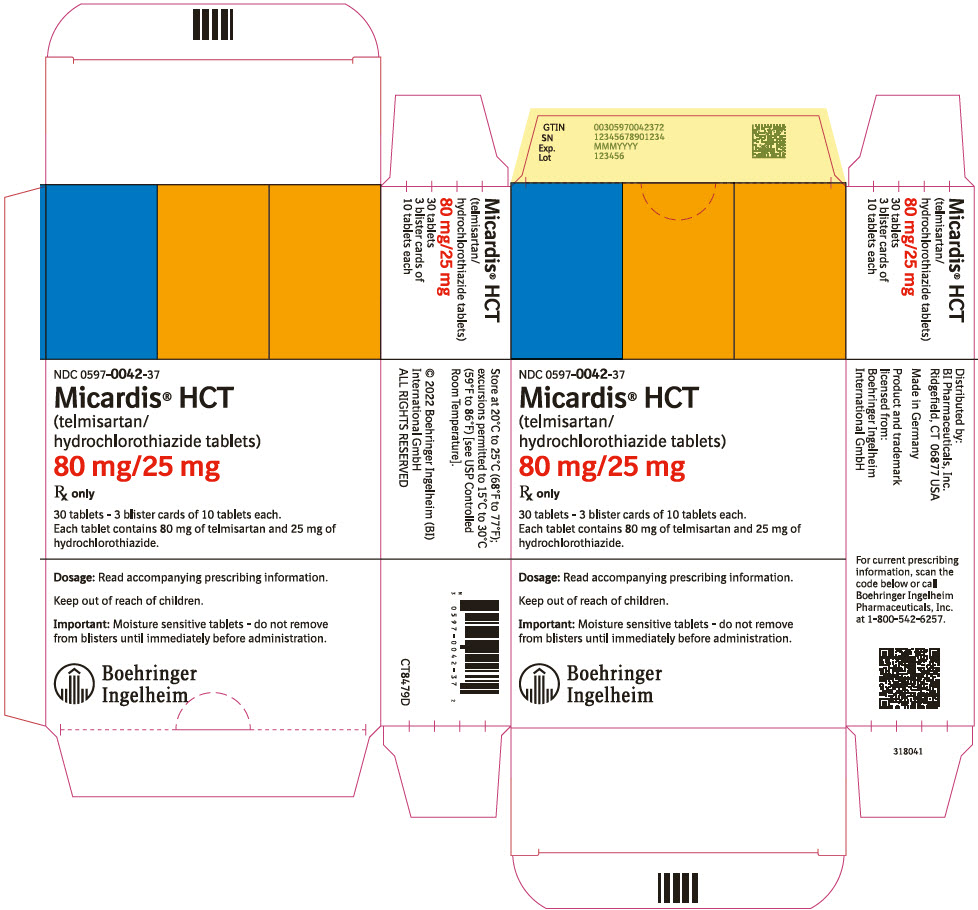
PRINCIPAL DISPLAY PANEL - 80 mg/25 mg Tablet Blister Card CartonNDC 0597-0042-37 - Micardis® HCT - (telmisartan/ hydrochlorothiazide tablets) 80 mg/25 mg - Rx only - 30 tablets - 3 blister cards of 10 tablets each. Each tablet contains 80 mg of telmisartan and 25 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information