Label: EZETIMIBE tablet
- NDC Code(s): 70518-3797-0, 70518-3797-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 16714-813
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EZETIMIBE TABLETS safely and effectively. See full prescribing information for EZETIMIBE TABLETS. EZETIMIBE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEEzetimibe tabletsareindicated: In combination with a statin, or alone when additional low-density lipoprotein cholesterol (LDL-C) lowering therapy isnot possible, as an adjunct to diet to ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of ezetimibe tablets is 10 mg orally once daily, administered with or without food. If as dose is missed, take the missed dose assoon as possible. Do not double the next ...
-
3 DOSAGE FORMS AND STRENGTHSEzetimibe tablets USP, 10 mg are white to off-white, capsule shaped beveled edge tablets debossed with "K 31" on one side and plain on other side.
-
4 CONTRAINDICATIONSEzetimibe tablets are contraindicated in patients with a known hypersensitivity to ezetimibe or any of the excipients in ezetimibe tablets. Hypersensitivity reactions including anaphylaxis ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risks Associated with Combination Treatment with a Statin, Fenofibrate, or Other LDL-C Lowering Therapies - If ezetimibe is administered with a statin, fenofibrate, orother LDL-C lowering ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the label: Liver enzyme abnormalities - [see - Warnings and Precautions (5.2) ...
-
7 DRUG INTERACTIONSTable 3 includes a list of drugs with clinically important drug interactions when administered concomitantly with ezetimibe and instructions for preventing or managing them. Table 3: Clinically ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient data on ezetimibe use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or ...
-
10 OVERDOSAGEIn the event of overdose, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
-
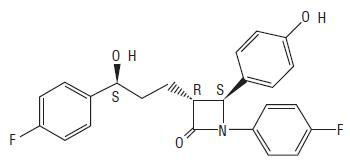
11 DESCRIPTIONEzetimibe is a dietary cholesterol absorption inhibitor. The chemical name of ezetimibe is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine. The molecular target of ezetimibe has been shown to be the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 104-week dietary carcinogenicity study with ezetimibe was conducted in rats at doses up to 1,500 mg/kg/day (males) and 500 mg/kg/day ...
-
14 CLINICAL STUDIESPrimary Hyperlipidemia in Adults - Ezetimibe reduces total-C, LDL-C, Apo B, and non-HDL-C in patients with hyperlipidemia. Maximal to near maximal response is generally achieved within 2 weeks ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEzetimibe Tablets USP, 10 mg white to off-white, capsule shaped beveled edge tablets debossed with "K 31" on one side and plain on other side. They are supplied as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-Approved Patient Labeling ( Patient Information). Inform patients that ezetimibe tablets may cause liver enzyme elevations - [see - Warnings and ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Ezetimibe (e zet' i mibe) tablets, for oral use - Read this information carefully before you start taking ezetimibe tablets and each time you get more ezetimibe ...
-
PRINCIPAL DISPLAY PANELDRUG: Ezetimibe - GENERIC: Ezetimibe - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-3797-0 - NDC: 70518-3797-1 - COLOR: white - SHAPE: CAPSULE - SCORE: No score - SIZE: 8 mm - IMPRINT: K;31 - PACKAGING: 90 in 1 ...
-
INGREDIENTS AND APPEARANCEProduct Information