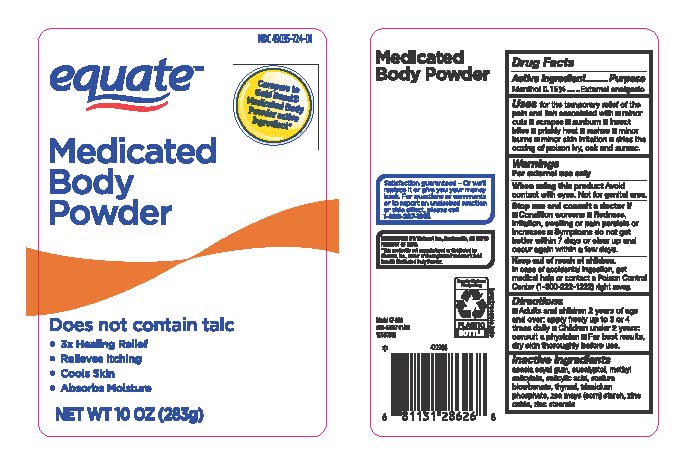
Label: MEDICATED BODY POWDER- menthol powder
- NDC Code(s): 79903-724-01, 79903-724-04
- Packager: Walmart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active Ingredients
- Purpose
- Uses
- Warnings
- When usin this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Equate Medicated Body Powder Original
-
INGREDIENTS AND APPEARANCE
MEDICATED BODY POWDER
menthol powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-724 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.42 g in 283 g Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) ACACIA (UNII: 5C5403N26O) EUCALYPTUS OIL (UNII: 2R04ONI662) MENTHYL SALICYLATE, (+/-)- (UNII: 43XOA705ZD) SALICYLIC ACID (UNII: O414PZ4LPZ) THYMOL (UNII: 3J50XA376E) ZINC STEARATE (UNII: H92E6QA4FV) ZINC OXIDE (UNII: SOI2LOH54Z) ZEA MAYS WHOLE (UNII: 1G5HNE09V8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-724-01 283 g in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 2 NDC:79903-724-04 113 g in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/01/2020 Labeler - Walmart Stores Inc (051957769)