Label: DIPHENHYDRAMINE HYDROCHLORIDE injection
- NDC Code(s): 0404-9851-01
- Packager: Henry Schein, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0641-0376
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
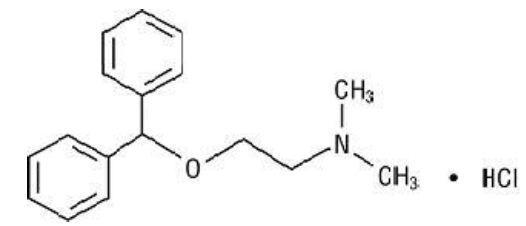
DESCRIPTIONDiphenhydramine Hydrochloride Injection is a sterile, nonpyrogenic solution for intravenous or deep intramuscular use as an antihistaminic agent. Each mL contains diphenhydramine hydrochloride 50 ...
-
CLINICAL PHARMACOLOGYDiphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector ...
-
INDICATIONS AND USAGEDiphenhydramine Hydrochloride Injection is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when the oral form is ...
-
CONTRAINDICATIONSUSE IN NEONATES OR PREMATURE INFANTS - This drug should not be used in neonates or premature infants. USE IN NURSING MOTHERS - Because of the higher risk of antihistamines for infants generally ...
-
WARNINGSAntihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy or ...
-
PRECAUTIONSGENERAL - Diphenhydramine hydrochloride has an atropine-like action and, therefore, should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure ...
-
ADVERSE REACTIONSThe most frequent adverse reactions are italicized. GENERAL - Urticaria; drug rash; anaphylactic shock; photosensitivity; excessive perspiration; chills; dryness of mouth, nose and ...
-
OVERDOSAGEAntihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in pediatric patients. Atropine-like signs and symptoms, dry ...
-
DOSAGE AND ADMINISTRATIONTHIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY. Diphenhydramine Hydrochloride Injection is indicated when the oral form is impractical. DOSAGE SHOULD BE INDIVIDUALIZED ...
-
HOW SUPPLIEDDiphenhydramine Hydrochloride Injection, USP 50 mg/mL - 1 mL vials packaged in 25s (NDC 0641-0376-25) Product repackaged by: Henry Schein, Inc., Bastian, VA 24314 - From Original ...
-
Sample Package Label

-
INGREDIENTS AND APPEARANCEProduct Information