Label: AMMONIA N 13- ammonia n-13 injection, solution
- NDC Code(s): 52670-552-30
- Packager: Mayo Clinic
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Ammonia N 13 Injection USPsafely and effectively. See full prescribing information forAmmonia N 13 Injection USP.
Ammonia N 13 Injection USP for intravenous use
Initial U.S. Approval: 2007INDICATIONS AND USAGE
Ammonia N 13 Injection USP is a radioactive diagnostic agent for Positron Emission Tomography (PET) indicated for diagnostic PET imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in patients with suspected or existing coronary artery disease ( 1).
DOSAGE AND ADMINISTRATION
Rest Imaging Study ( 2.1):
- Aseptically withdraw Ammonia N13 Injection from its container and administer 0.368-0.736 GBq (10-20 mCi) as a bolus through a catheter inserted into a large peripheral vein.
- Start imaging 3 minutes after the injection and acquire images for a total of 10-20 minutes.
- Stress Imaging Study (
2.2):
- If a rest imaging study is performed, begin the stress imaging study 40 minutes or more after the first Ammonia N 13 Injection USP to allow sufficient isotope decay.
- Administer a pharmacologic stress-inducing drug in accordance with its labeling.
- Aseptically withdraw Ammonia N 13 Injection USP from its container and administer 0.368-0.736 GBq (10-20 mCi) of Ammonia N 13 Injection USP as a bolus at 8 minutes after the administration of the pharmacologic stress-inducing drug.
- Start imaging 3 minutes after the Ammonia N 13 Injection USP and acquire Images for a total of 10-20 minutes.
Patient Preparation ( 2.3):
- To increase renal clearance of radioactivity and to minimize radiation dose to the bladder, hydrate the patient before the procedure and encourage voiding as soon as each image acquisition is completed and as often as possible thereafter for at least one hour.
DOSAGE FORMS AND STRENGTHS
Glass vial containing 0.138-1.387 GBq/mL (3.75-37.5 mCi/mL) of Ammonia N 13 Injection USP in aqueous 0.9% sodium chloride solution (approximately 6 mL volume) ( 3).
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
Ammonia N 13 Injection USP may increase the risk of cancer. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker ( 5).
ADVERSE REACTIONS
No adverse reactions have been reported for Ammonia N 13 Injection USP based on a review of the published literature, publicly available reference sources, and adverse drug reaction reporting system ( 6).
To report SUSPECTED ADVERSE REACTIONS, contact Division of Nuclear Medicine, Department of Radiology, Mayo Clinic at 507-284-2511 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
- It is not known whether this drug is excreted in human milk. Alternatives to breastfeeding (e.g. using stored breast milk or infant formula) should be used for 2 hours (> 10 half-lives of radioactive decay for N 13 isotope) after administration of Ammonia N 13 Injection USP (
8.3).
- The safety and effectiveness of Ammonia N 13 Injection USP has been established in pediatric patients ( 8.4).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2014
- Aseptically withdraw Ammonia N13 Injection from its container and administer 0.368-0.736 GBq (10-20 mCi) as a bolus through a catheter inserted into a large peripheral vein.
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Rest Imaging Study
2.2 Stress Imaging Study
2.3 Patient Preparation
2.4 Radiation Dosimetry
2.5 Drug Handling
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risks
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment o f Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage
17 PATIENT COUNSELING INFORMATION
17.1 Pre-study Hydration
17.2 Post-study Voiding
17.3 Post-study Breastfeeding Avoidance
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Rest Imaging Study
- Aseptically withdraw Ammonia N 13 Injection USP from its container and administer 0.368-0.736 GBq (10-20 mCi) as a bolus through a catheter inserted into a large peripheral vein.
- Start imaging 3 minutes after the injection and acquire images for a total of 10-20 minutes.
2.2 Stress Imaging Study
- If a rest imaging study is performed, begin the stress imaging study 40 minutes or more after the first Ammonia N 13 Injection USP to allow sufficient isotope decay.
- Administer a pharmacologic stress-inducing drug in accordance with its labeling.
- Aseptically withdraw Ammonia N 13 Injection USP from its container and administer 0.368-0.736 GBq (10-20 mCi) of Ammonia N 13 Injection USP as a bolus at 8 minutes after the administration of the pharmacologic stress-inducing drug. Start imaging 3 minutes after the Ammonia N 13 Injection USP and acquire images for a total of 10-20 minutes.
2.3 Patient Preparation
To increase renal clearance of radioactivity and to minimize radiation dose to the bladder, ensure that the patient is well hydrated before the procedure and encourage voiding as soon as a study is completed and as often as possible thereafter for at least one hour.
2.4 Radiation Dosimetry
The converted radiation absorbed doses in rem/mCi are shown in Table 1. These estimates are calculated from the Task Group of Committee 2 of the International Commission on Radiation Protection. 1
Table 1: N 13 Absorbed Radiation Dose Per Unit Activity (rem/mCi) for Adults and Pediatric Groups. Organ Age (years) Adult 15 10 5 1 Adrenals 0.0085 0.0096 0.016 0.025 0.048 Bladder wall 0.030 0.037 0.056 0.089 0.17 Bone surfaces 0.0059 0.0070 0.011 0.019 0.037 Brain 0.016 0.016 0.017 0.019 0.027 Breast 0.0067 0.0067 0.010 0.017 0.033 Stomach wall 0.0063 0.0078 0.012 0.019 0.037 Small intestine 0.0067 0.0081 0.013 0.021 0.041 *ULI 0.0067 0.0078 0.013 0.021 0.037 **LLI 0.0070 0.0078 0.013 0.020 0.037 Heart 0.0078 0.0096 0.015 0.023 0.041 Kidneys 0.017 0.021 0.031 0.048 0.089 Liver 0.015 0.018 0.029 0.044 0.085 Lungs 0.0093 0.011 0.018 0.029 0.056 Ovaries 0.0063 0.0085 0.014 0.021 0.041 Pancreas 0.0070 0.0085 0.014 0.021 0.041 Red marrow 0.0063 0.0078 0.012 0.020 0.037 Spleen 0.0093 0.011 0.019 0.030 0.056 Testes 0.0067 0.0070 0.011 0.018 0.035 Thyroid 0.0063 0.0081 0.013 0.021 0.041 Uterus 0.0070 0.0089 0.014 0.023 0.041 Other tissues 0.0059 0.0070 0.011 0.018 0.035 *Upper large intestine, ** Lower large intestine
2.5 Drug Handling
- Inspect Ammonia N 13 Injection USP visually for particulate matter and discoloration before administration, whenever solution and container permit.
- Do not administer Ammonia N 13 Injection USP containing particulate matter or discoloration; dispose of these unacceptable or unused preparations in a safe manner, in compliance with applicable regulations.
- Wear sterile gloves and effective shielding when handling Ammonia N 13 Injection USP.
- Use aseptic technique to maintain sterility during all operations involved in the manipulation and administration of Ammonia N 13 Injection USP. The contents of each vial are sterile and non-pyrogenic.
- Use appropriate safety measures, including shielding, consistent with proper patient management to avoid unnecessary radiation exposure to the patient, occupational workers, clinical personnel, and other persons.
- Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
- Before administration of Ammonia N 13 Injection USP, assay the dose in a properly calibrated dose calibrator.
- Aseptically withdraw Ammonia N 13 Injection USP from its container and administer 0.368-0.736 GBq (10-20 mCi) as a bolus through a catheter inserted into a large peripheral vein.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risks
Ammonia N 13 Injection USP may increase the risk of cancer. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker [see Dosage and Administration (2.4)].
- 6 ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Ammonia N 13 Injection USP. It is also not known whether Ammonia N 13 Injection USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Ammonia N 13 Injection USP should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for radiation exposure to nursing infants from Ammonia N 13 Injection USP, use alternative infant nutrition sources (e.g. stored breast milk or infant formula) for 2 hours (>10 half-lives of radioactive decay for N 13 isotope) after administration of the drug or avoid use of the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of Ammonia N 13 Injection USP has been established in pediatric patients based on known metabolism of ammonia, radiation dosimetry in the pediatric population, and clinical studies in adults [see Dosage and Administration (2.4)].
-
11 DESCRIPTION
11.1 Chemical Characteristics
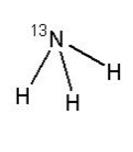
Ammonia N 13 Injection USP is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging. The active ingredient, [ 13N] ammonia, has the molecular formula of 13NH 3 with a molecular weight of 16.02g and has the following chemical structure:
Ammonia N 13 Injection USP is provided as a ready to use sterile, pyrogen-free, clear and colorless solution. Each mL of the solution contains between 0.138 GBq to 1.387 GBQ (3.75 mCi to 37.5mCi) of [ 13N] ammonia, at the end of synthesis (EOS), in 0.9% aqueous sodium chloride. The pH of the solution is between 4.5 to 7.5. The recommended dose of radioactivity (0.368-0.736 GBq, 10-20 mCi) is associated with a theoretical mass dose of 0.5-1.0 picomoles (8.47-16.94 picograms) of ammonia.
11.2 Physical Characteristics
Nitrogen N 13 decays by emitting positron to Carbon C 13 (stable) and has a physical half-life of 9.96 minutes. The principal photons useful for imaging are the dual 511 keV gamma photons that are produced and emitted simultaneously in opposite direction when the positron interacts with an electron (Table 2).
Table 2: Principal Radiation Emission Data for Nitrogen 13 Radiation/Emission % Per Disintegration Energy Positron(β+)
100 1190 keV (Max.) Gamma(±)*
200 511 keV *Produced by positron annihilation
The specific gamma ray constant (point source air kerma coefficient) for nitrogen N13 is 1.39 x 10 -6 Gy/hr/kBq (5.9 R/hr/mCi) at 1 cm. The half-value layer (HVL) of lead (Pb) for 511 keV photons is 4 mm. Selected coefficients of attenuation are listed in Table 3 as a function of lead shield thickness. For example, the use of 39 mm thickness of lead will attenuate the external radiation by a factor of about 1000.
Table 3: Radiation Attenuation of 511 keV Photons by lead (Pb) shielding Shield Thickness (Pb) mm Coefficient of Attenuation 4 0.5 8 0.25 13 0.1 26 0.01 39 0.001 52 0.0001 Table 4 lists fractions remaining at selected time intervals from the calibration time. This information may be used to correct for physical decay of the radionuclide.
Table 4: Physical Decay Chart for Nitrogen N 13 Minutes Fraction Remaining 0* 1.000 5 0.706 10 0.499 15 0.352 20 0.249 25 0.176 30 0.124 *Calibration time
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ammonia N 13 Injection USP is a radiolabeled analog of ammonia that is distributed to all organs of the body after intravenous administration. It is extracted from the blood in the coronary capillaries into the myocardial cells where it is metabolized to glutamine N 13 and retained in the cells. The presence of ammonia N 13 and glutamine N 13 in the myocardium allows for PET imaging of the myocardium.
12.2 Pharmacodynamics
Following intravenous injection, ammonia N 13 enters the myocardium through the coronary arteries. The PET technique measures myocardial blood flow based on the assumption of a three-compartmental disposition of intravenous ammonia N 13 in the myocardium. In this model, the value of the rate constant, which represents the delivery of blood to myocardium, and the fraction of ammonia N 13 extracted into the myocardial cells, is a measure of myocardial blood flow. Optimal PET imaging of the myocardium is generally achieved between 10 to 20 minutes after administration.
12.3 Pharmacokinetics
Following intravenous injection, Ammonia N 13 Injection USP is cleared from the blood with a biologic half-life of about 2.84 minutes (effective half-life of about 2.21 minutes). In the myocardium, its biologic half-life has been estimated to be less than 2 minutes (effective half-life less than 1.67 minutes).
The mass dose of Ammonia N 13 Injection USP is very small as compared to the normal range of ammonia in the blood (0.72-3.30 mg) in a healthy adult man [see Description (11.1)].
Plasma protein binding of ammonia N 13 or its N 13 metabolites has not been studied.
Ammonia N 13 undergoes a five-enzyme step metabolism in the liver to yield urea N 13 (the main circulating metabolite). It is also metabolized to glutamine N 13 (the main metabolite in tissues) by glutamine synthesis in the skeletal muscles, liver, brain, myocardium, and other organs. Other metabolites of ammonia N 13 include small amounts of N 13 amino acid anions (acidic amino acids) in the forms of glutamate N 13 or aspartate N 13.
Ammonia N 13 is eliminated from the body by urinary excretion mainly as urea N 13.
The pharmacokinetics of Ammonia N 13 Injection USP have not been studied in renally impaired, hepatically impaired, or pediatric patients.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment o f Fertility
Long term animal studies have not been performed to evaluate the carcinogenic potential of Ammonia N 13 Injection USP. Genotoxicity assays and impairment of male and female fertility studies with Ammonia N 13 Injection USP have not been performed.
-
14 CLINICAL STUDIES
In a descriptive, prospective, blinded image interpretation study 2 of adult patients with known or suspected coronary artery disease, myocardial perfusion deficits in stress and rest PET images obtained with Ammonia N 13 (N=111) or Rubidium 82 (N=82) were compared to changes in stenosis flow reserve (SFR) as determined by coronary angiography. The principal outcome of the study was the evaluation of PET defect severity relative to SFR.
PET perfusion defects at rest and stress for seven cardiac regions (anterior, apical, anteroseptal, posteroseptal, anterolateral, posterolateral, and inferior walls) were graded on a 0 to 5 scale defined as normal (0), possible (1), probable (2), mild (3), moderate (4), and severe (5) defects. Coronary angiograms were used to measure absolute and relative stenosis dimensions and to calculate stenosis flow reserve defined as the maximum value of flow at maximum coronary vasodilatation relative to rest flow under standardized hemodynamic conditions. SFR scores ranged from 0 (total occlusion) to 5 (normal).
With increasing impairment of flow reserve, the subjective PET defect severity increased. A PET defect score of 2 or higher was positively correlated with flow reserve impairment (SFR<3).
-
15 REFERENCES
1Annals of the ICRP. Publication 53. Radiation dose to patients from radiopharmaceuticals. New York: Pergamon Press, 1988.
2Demer, L.L.K.L.Gould, R.A.Goldstein, R.L.Kirkeeide, N.A.Mullani, R.W. Smalling, A.Nishikawa, and M.E.Merhige. Assessment of coronary artery disease severity by PET: Comparison with quantitative arteriography in 193 patients. Circulation 1989; 79: 825-35.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ammonia N 13 Injection USP is packaged in 30 mL single dose glass vial containing between 1.11 GBq to 11.1 GBq (30 mCi to 300 mCi) of [ 13N] ammonia, at the end of synthesis (EOS), in 0.9% sodium chloride injection solution in approximately 6 mL volume. The recommended dose of radioactivity (0.368-0.736 GBq, 10-20 mCi) is associated with a theoretical mass dose of 0.5-1.0 picomoles (8.47-16.94 picograms) of ammonia.
-
17 PATIENT COUNSELING INFORMATION
17.1 Pre-study Hydration
Instruct patients to drink plenty of water or other fluids (as tolerated) in the 4 hours before their PET study.
17.2 Post-study Voiding
Instruct patients to void after completion of each image acquisition session and as often as possible for one hour after the PET scan ends.
17.3 Post-study Breastfeeding Avoidance
Instruct nursing patients to substitute stored breast milk or infant formula for breast milk for 2 hours after administration of Ammonia N 13 Injection USP.
Manufactured by:
Mayo Clinic PET Radiochemistry Facility
200 1 st St SW
Rochester, MN 55905Mayo Clinic PET Radiochemistry Facility
5861 E Mayo Blvd
Phoenix, AZ 85054Distributed by:
Mayo Clinic PET Radiochemistry Facility
200 1 st St SW
Rochester, MN 55905Mayo Clinic PET Radiochemistry Facility
5861 E Mayo Blvd
Phoenix, AZ 85054 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMMONIA N 13
ammonia n-13 injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52670-552 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA N-13 (UNII: 9OQO0E343Z) (AMMONIA N-13 - UNII:9OQO0E343Z) AMMONIA N-13 37.5 mCi in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52670-552-30 30 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 02/25/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203321 02/25/2013 Labeler - Mayo Clinic (167141923) Establishment Name Address ID/FEI Business Operations Mayo Clinic PET Radiochemistry Facility 080416065 manufacture(52670-552) Establishment Name Address ID/FEI Business Operations Mayo Clinic PET Radiochemistry Facility 080502087 manufacture(52670-552) Establishment Name Address ID/FEI Business Operations Mayo Clinic PET Radiochemistry Facility 081008297 manufacture(52670-552)


