Label: ZUNVEYL- benzgalantamine tablet, delayed release
- NDC Code(s): 84054-005-60, 84054-010-60, 84054-015-60
- Packager: Alpha Cognition, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 18, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZUNVEYL safely and effectively. See full prescribing information for ZUNVEYL. ZUNVEYL (benzgalantamine) delayed-release tablets ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZUNVEYL is indicated for the treatment of mild to moderate dementia of the Alzheimer's type in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage and Administration - Dosage - The recommended starting dosage is 5 mg twice a day (10 mg/day) by mouth. Increase to initial maintenance dosage of 10 mg twice daily (20 ...
-
3 DOSAGE FORMS AND STRENGTHSZUNVEYL delayed-release tablets are enteric coated and available in the following strengths of benzgalantamine: 5 mg white, round, and convex, debossed with "B05" in grey - 10 mg purple, round, and ...
-
4 CONTRAINDICATIONSZUNVEYL is contraindicated in patients with known hypersensitivity to benzgalantamine, galantamine, or to any inactive ingredient in ZUNVEYL. Serious skin reactions have occurred [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Skin Reactions - Serious skin reactions (e.g., Stevens-Johnson syndrome and acute generalized exanthematous pustulosis) have been reported in patients receiving galantamine (the ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more detail elsewhere in the labeling: Serious Skin Reactions [see Warnings and Precautions (5.1)] Cardiovascular Conditions [see ...
-
7 DRUG INTERACTIONS7.1 Use with Anticholinergics - Galantamine has the potential to interfere with the activity of anticholinergic medications. 7.2 Use with Cholinomimetics and Other Cholinesterase Inhibitors - A ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of ZUNVEYL or galantamine in pregnant women. In studies conducted in animals ...
-
10 OVERDOSAGEBecause strategies for the management of overdose are continually evolving, it is advisable to contact a poison control center to determine the latest recommendations for the management of an ...
-
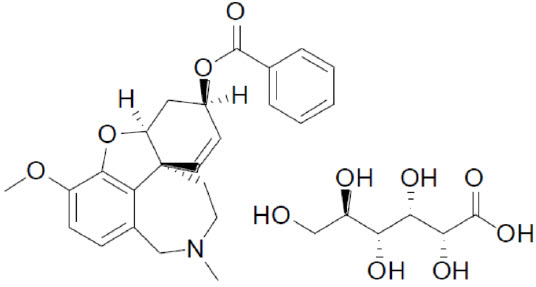
11 DESCRIPTIONZUNVEYL (benzgalantamine) is a prodrug of galantamine, an acetylcholinesterase inhibitor . Benzgalantamine gluconate is known chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Although the etiology of cognitive impairment in Alzheimer's disease (AD) is not fully understood, it has been reported that acetylcholine-producing neurons degenerate ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 24-month oral carcinogenicity study in rats, an increase in endometrial adenocarcinomas was observed at 10 ...
-
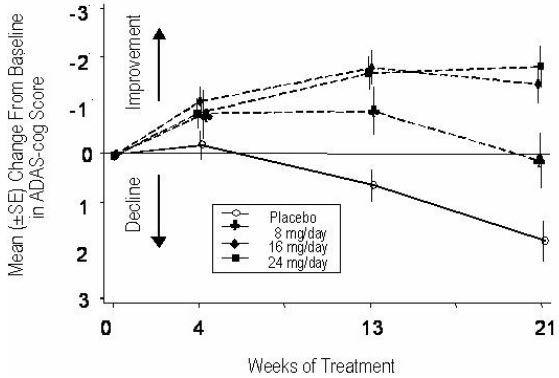
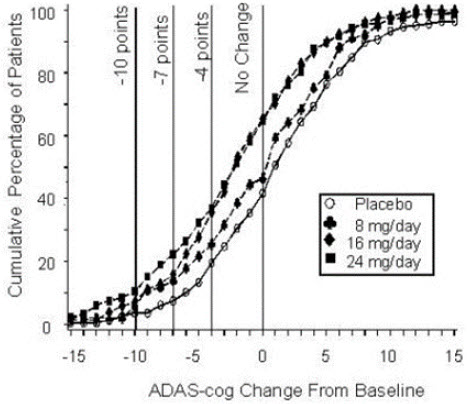
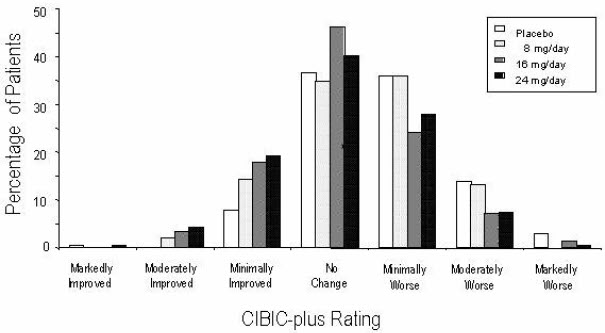
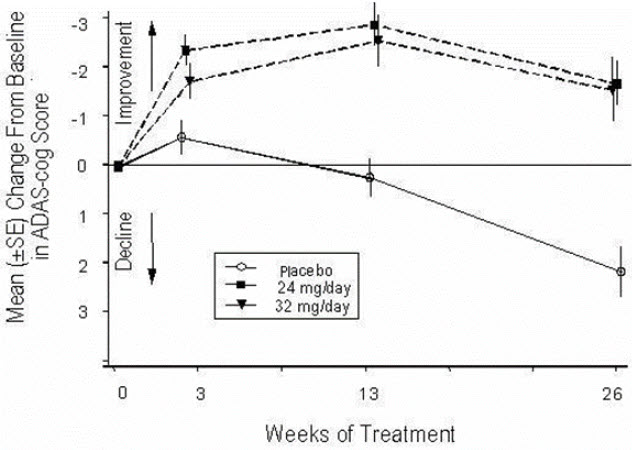
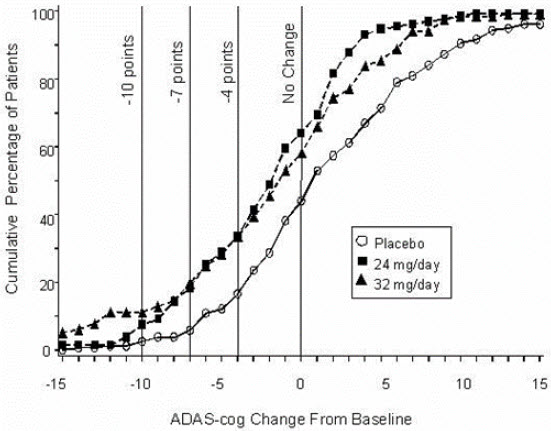
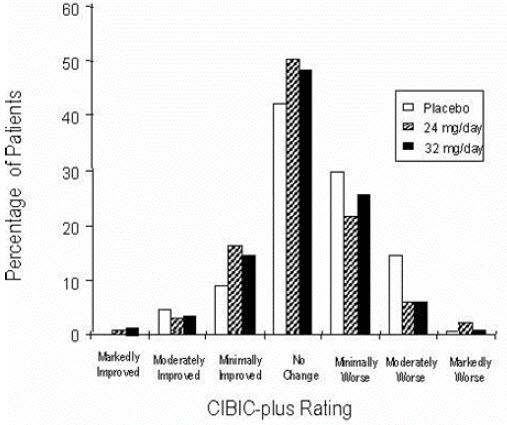
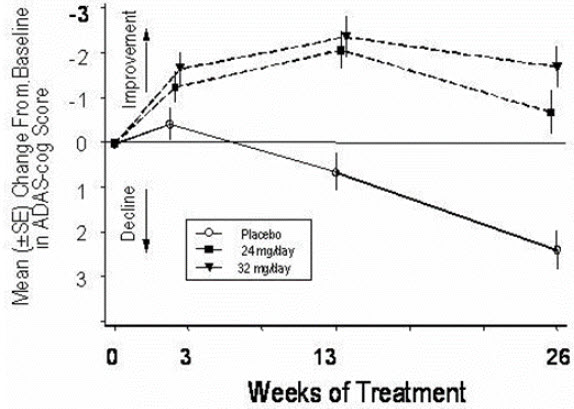
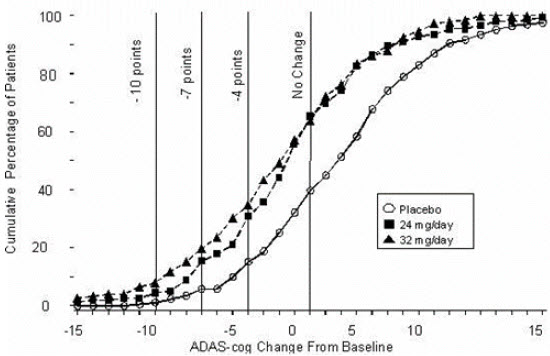
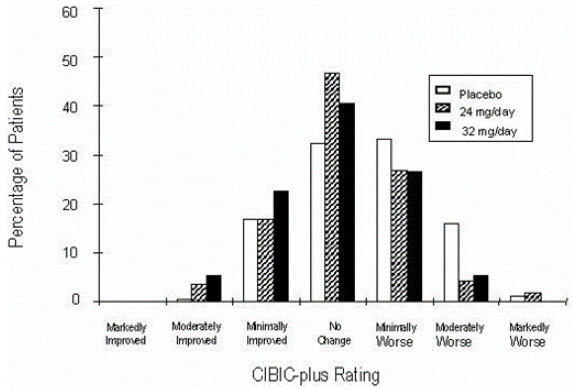
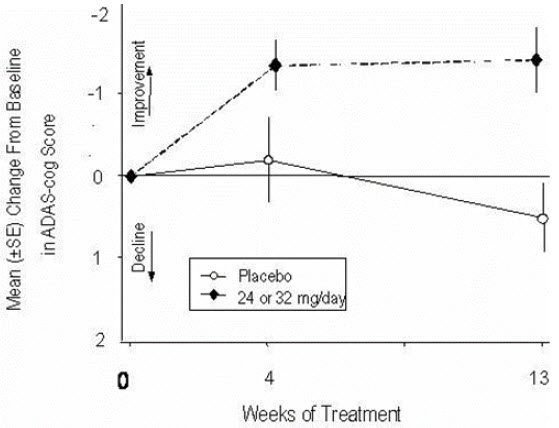
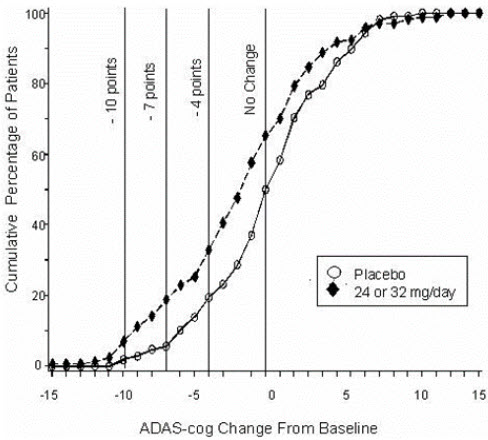
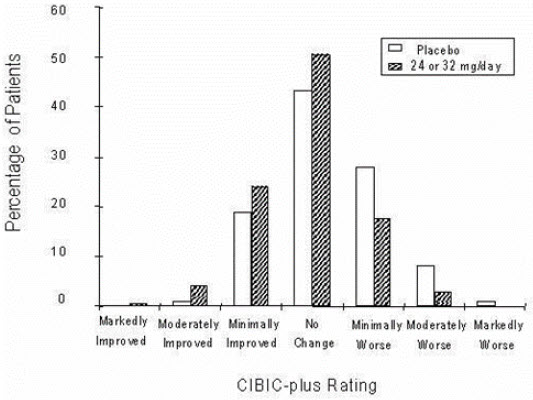
14 CLINICAL STUDIESThe efficacy of ZUNVEYL is based upon 3 bioavailability studies in healthy adults comparing galantamine immediate-release tablets and galantamine extended-release capsules to ZUNVEYL [see Clinical ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ZUNVEYL delayed-release tablets are enteric coated and supplied in child-resistant packages as follows: 5 mg white tablet debossed with "B05" in grey – bottles of 60 (NDC ...
-
17 PATIENT COUNSELING INFORMATIONSerious Skin Reactions - Advise patients and caregivers to discontinue ZUNVEYL and seek immediate medical attention at the first appearance of skin rash [see Warnings and Precautions ...
-
SPL UNCLASSIFIED SECTIONZUNVEYL delayed-release tablets are manufactured for: Alpha Cognition, Inc. 1452 Hughes Rd - Suite 200 - Grapevine, TX 76051
-
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle LabelNDC 84054 -005-60 - Rx Only - ZUNVEYL - (benzgalantamine) DELAYED RELEASE TABLETS - 5 mg - 60 Tablets
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle LabelNDC 84054 -010-60 - Rx Only - ZUNVEYL - (benzgalantamine) DELAYED RELEASE TABLETS - 10 mg - 60 Tablets
-
PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle LabelNDC 84054 -015-60 - Rx Only - ZUNVEYL - (benzgalantamine) DELAYED RELEASE TABLETS - 15 mg - 60 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information