Label: XATMEP- methotrexate solution
- NDC Code(s): 52652-2001-1, 52652-2001-6
- Packager: Azurity Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XATMEP® safely and effectively. See full prescribing information for XATMEP.
XATMEP (methotrexate) oral solution
Initial U.S. Approval: 1953WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
- •
- Methotrexate can cause severe or fatal toxicities. Monitor closely and modify dose or discontinue for the following toxicities: bone marrow suppression (5.1), infection (5.2), renal (5.3), gastrointestinal (5.4), hepatic (5.5), pulmonary (5.6), hypersensitivity and dermatologic (5.7).
- •
- Methotrexate can cause embryo-fetal toxicity and fetal death. Use in polyarticular juvenile idiopathic arthritis is contraindicated in pregnancy (4). Consider the benefits and risks of XATMEP and risks to the fetus when prescribing XATMEP to a pregnant patient with a neoplastic disease. Advise patients to use effective contraception during and after treatment with XATMEP (5.9, 8.1, 8.3).
INDICATIONS AND USAGE
XATMEP is a folate analog metabolic inhibitor indicated for the:
- •
- Treatment of pediatric patients with acute lymphoblastic leukemia (ALL) as a component of a combination chemotherapy maintenance regimen (1.1).
- •
- Management of pediatric patients with active polyarticular juvenile idiopathic arthritis (pJIA) who are intolerant of or had an inadequate response to first-line therapy (1.2).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Oral solution: 2.5 mg/mL (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Secondary malignancies can occur. In case of immunosuppression-associated lymphoma, discontinue methotrexate before starting treatment for lymphoma (5.8).
- •
- Immunizations may be ineffective (5.10).
- •
- Effects on reproduction: May cause impairment of fertility, oligospermia and menstrual dysfunction (5.11, 8.3).
ADVERSE REACTIONS
Most common adverse reactions are: ulcerative stomatitis, leukopenia, nausea, abdominal distress, and elevated liver function tests. Other frequently reported adverse reactions are malaise, fatigue, chills and fever, dizziness and decreased resistance to infection (6).
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc., at 1-855-379-0383 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Oral Antibiotics: May increase hematologic and gastrointestinal toxicity. Monitor patients accordingly (7.1).
- •
- Nitrous Oxide: May increase the risk of toxicity (7.1).
- •
- NSAIDs, Aspirin, and Steroids: May elevate and prolong serum methotrexate levels and increase gastrointestinal toxicity. Monitor patients accordingly (7.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Acute Lymphoblastic Leukemia
1.2 Polyarticular Juvenile Idiopathic Arthritis
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Acute Lymphoblastic Leukemia
2.3 Polyarticular Juvenile Idiopathic Arthritis
2.4 Evaluations Prior to Starting Methotrexate
2.5 Handling Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
5.2 Serious Infections
5.3 Renal Toxicity and Increased Toxicity with Renal Impairment
5.4 Gastrointestinal Toxicity
5.5 Hepatic Toxicity
5.6 Pulmonary Toxicity
5.7 Hypersensitivity and Dermatologic Reactions
5.8 Secondary Malignancies
5.9 Embryo-Fetal Toxicity
5.10 Ineffective Immunization and Risks Associated with Live Vaccines
5.11 Effects on Reproduction
5.12 Increased Toxicity Due to Third-Space Accumulation
5.13 Soft Tissue and Bone Toxicity with Radiation Therapy
5.14 Laboratory Tests
5.15 Risk of Improper Dosing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on XATMEP
7.2 Effect of XATMEP on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY
Methotrexate can cause the following severe or fatal adverse reactions. Monitor closely and modify dose or discontinue methotrexate as appropriate.
- •
- Bone marrow suppression [see Warnings and Precautions (5.1)]
- •
- Serious infections [see Warnings and Precautions (5.2)]
- •
- Renal toxicity and increased toxicity with renal impairment [see Warnings and Precautions (5.3)]
- •
- Gastrointestinal toxicity [see Warnings and Precautions (5.4)]
- •
- Hepatic toxicity [see Warnings and Precautions (5.5)]
- •
- Pulmonary toxicity [see Warnings and Precautions (5.6)]
- •
- Hypersensitivity and dermatologic reactions [see Warnings and Precautions (5.7)]
- •
- Methotrexate can cause embryo-fetal toxicity, including fetal death. Use in pJIA is contraindicated in pregnancy. Consider the benefits and risks of XATMEP and risks to the fetus when prescribing XATMEP to a pregnant patient with a neoplastic disease. Advise females and males of reproductive potential to use effective contraception during and after treatment with XATMEP [see Contraindications (4), Warnings and Precautions (5.9), Use in Specific Populations (8.1, 8.3)].
-
1 INDICATIONS AND USAGE
1.1 Acute Lymphoblastic Leukemia
XATMEP is indicated for the treatment of pediatric patients with acute lymphoblastic leukemia (ALL) as part of a multi-phase, combination chemotherapy maintenance regimen.
1.2 Polyarticular Juvenile Idiopathic Arthritis
XATMEP is indicated in the management of pediatric patients with active polyarticular juvenile idiopathic arthritis (pJIA) who have had an insufficient therapeutic response to, or are intolerant of, an adequate trial of first-line therapy including full dose non-steroidal anti-inflammatory agents (NSAIDs).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
XATMEP is intended for oral use only. Use another formulation of methotrexate for alternative dosing in patients who require dosing via other routes of administration. Instruct patients and caregivers that the recommended dose should be taken weekly, as directed, and that mistaken daily use of the recommended dose has led to fatal toxicity [see Warnings and Precautions (5.15), Overdosage (10)].
It is important that XATMEP be measured with an accurate measuring device [see Warnings and Precautions (5.15), Patient Counseling Information (17)]. A household teaspoon is not an accurate measuring device. A pharmacist can provide an appropriate device and can provide instructions for measuring the correct dose.
2.2 Acute Lymphoblastic Leukemia
The recommended starting dose of XATMEP, in multi-agent combination chemotherapy maintenance regimens, is 20 mg/m2 given one time weekly. After initiating XATMEP, continuation of appropriate dosing requires periodic monitoring of absolute neutrophil count (ANC) and platelet count to assure sufficient drug exposure (that is to maintain ANC at a desirable level) and to adjust for excessive hematological toxicity.
2.3 Polyarticular Juvenile Idiopathic Arthritis
The recommended starting dose of XATMEP is 10 mg/m2 given one time weekly.
Dosages should be tailored to the individual patient and adjusted gradually to achieve an optimal response. Although there is experience with doses up to 30 mg/m2/week in pediatric patients, doses greater than 20 mg/m2/week may result in a significant increase in the incidence and severity of serious toxic reactions, especially bone marrow suppression. Doses between 20 and 30 mg/m2/week (0.65 to 1 mg/kg/week) may have better absorption and fewer gastrointestinal side effects if methotrexate is administered by an alternative route using another formulation.
Therapeutic response usually begins within 3 to 6 weeks and the patient may continue to improve for another 12 weeks or more.
Certain side effects such as mouth sores may be reduced by folate supplementation with methotrexate in pJIA.
2.4 Evaluations Prior to Starting Methotrexate
Assess hematologic, hepatic, and renal function before beginning, as well as periodically during and before reinstituting, therapy with XATMEP [see Warnings and Precautions (5.1, 5.3, 5.5, 5.7, 5.14)]. Exclude pregnancy in females of reproductive potential before starting XATMEP [see Contraindications (4), Warnings and Precautions (5.9), Use in Specific Populations (8.1, 8.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
XATMEP is contraindicated in the following:
- •
- Pregnancy in patients with non-malignant diseases. XATMEP can cause embryo-fetal toxicity and fetal death when administered during pregnancy [see Warnings and Precautions (5.9), Use in Specific Populations (8.1)].
- •
- Patients with severe hypersensitivity to methotrexate [see Warnings and Precautions (5.7), Adverse Reactions (6.1, 6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
XATMEP suppresses hematopoiesis and can cause severe and life-threatening pancytopenia, anemia, leukopenia, neutropenia, and thrombocytopenia.
Obtain blood counts at baseline and periodically during treatment. Monitor patients for possible clinical complications of bone marrow suppression. Provide supportive care and modify dose or discontinue XATMEP as needed.
5.2 Serious Infections
Patients treated with XATMEP are at increased risk for developing life-threatening or fatal bacterial, fungal, or viral infections including opportunistic infections such as Pneumocystis jiroveci pneumonia, invasive fungal infections, hepatitis B reactivation, tuberculosis primary infection or reactivation, and disseminated Herpes zoster and cytomegalovirus infections.
Monitor patients for the signs and symptoms of infection during and after treatment with XATMEP and treat promptly. Consider dose modification or discontinuation of XATMEP in patients who develop serious infections [see Warnings and Precautions (5.1)].
5.3 Renal Toxicity and Increased Toxicity with Renal Impairment
XATMEP can cause renal damage including acute renal failure. Monitor renal function to decrease the risk of renal injury and mitigate renal toxicity.
Consider administration of glucarpidase in patients with toxic plasma methotrexate concentrations (> 1 micromole per liter) and delayed clearance due to impaired renal function [see glucarpidase Prescribing Information].
5.4 Gastrointestinal Toxicity
XATMEP can cause diarrhea, vomiting, stomatitis, hemorrhagic enteritis, and fatal intestinal perforation. Patients with peptic ulcer disease or ulcerative colitis are at a greater risk of developing severe gastrointestinal adverse reactions.
Interrupt or discontinue XATMEP and institute appropriate supportive care as needed.
Unexpectedly severe and fatal gastrointestinal toxicity can occur with concomitant administration of XATMEP (primarily at high dosage) and nonsteroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.1)].
5.5 Hepatic Toxicity
XATMEP can cause severe and potentially irreversible hepatotoxicity including fibrosis, cirrhosis, and fatal liver failure. Avoid use of XATMEP in patients with chronic liver disease.
Assess liver function prior to initiating XATMEP and monitor liver function tests during treatment. Interrupt or discontinue XATMEP as appropriate. Transient asymptomatic acute liver enzyme elevations are common and are not predictive of subsequent hepatic disease. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis.
Other risk factors for hepatotoxicity include alcoholism, obesity, diabetes, hyperlipidemia, previous significant exposure to liver toxins, history of liver disease, family history of inheritable liver disease, persistent abnormal liver chemistry findings, duration of therapy, and advanced age.
5.6 Pulmonary Toxicity
Methotrexate-induced pulmonary toxicity including acute or chronic interstitial pneumonitis and irreversible or fatal cases can occur at all dose levels. Monitor patients for signs of pulmonary toxicity and interrupt or discontinue XATMEP as appropriate.
5.7 Hypersensitivity and Dermatologic Reactions
Severe, including fatal, dermatologic reactions, such as toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, skin necrosis, erythema multiforme, can occur with methotrexate. Discontinue XATMEP if severe dermatologic reactions occur.
Anaphylaxis can occur with methotrexate. If anaphylaxis or any other serious hypersensitivity reaction occurs, immediately discontinue methotrexate and institute appropriate therapy. Methotrexate is contraindicated for use in patients with a history of severe hypersensitivity.
Radiation dermatitis and sunburn may be “recalled” by the use of methotrexate.
5.8 Secondary Malignancies
Secondary malignancies can occur at all dose levels of methotrexate.
There have been instances of lymphoproliferative disease associated with low-dose oral methotrexate which have regressed completely following withdrawal of methotrexate without institution of antineoplastic therapy. Discontinue XATMEP first and institute appropriate treatment if the lymphoma does not regress.
5.9 Embryo-Fetal Toxicity
Based on published reports and methotrexate’s mechanism of action, methotrexate can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman. In pregnant women with non-malignant diseases, methotrexate is contraindicated. Consider the benefits and risks of XATMEP and risks to the fetus when prescribing XATMEP to a pregnant patient with a neoplastic disease. Advise females of reproductive potential to use effective contraception during therapy and for 6 months after the final dose. Advise males of reproductive potential to use effective contraception during and for at least 3 months after the final methotrexate dose [see Contraindications (4), Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
5.10 Ineffective Immunization and Risks Associated with Live Vaccines
Immunization may be ineffective when given during XATMEP therapy.
Immunization with live virus vaccines is not recommended. There have been reports of disseminated vaccinia infections after smallpox immunization in patients receiving methotrexate therapy.
5.11 Effects on Reproduction
Based on published reports, methotrexate can cause impairment of fertility, oligospermia, and menstrual dysfunction. It is not known if the infertility is reversible in affected patients. Discuss the risk of effects on reproduction with female and male patients [see Use in Specific Populations (8.3)].
5.12 Increased Toxicity Due to Third-Space Accumulation
Methotrexate can exit slowly from third‑space accumulations resulting in prolonged terminal plasma half-life and toxicity. Evacuate significant third-space accumulations prior to methotrexate administration [see Clinical Pharmacology (12.3)].
5.13 Soft Tissue and Bone Toxicity with Radiation Therapy
Concomitant radiation therapy increases the risk of soft tissue necrosis and osteonecrosis associated with methotrexate.
5.14 Laboratory Tests
Closely monitor patients undergoing XATMEP therapy so that toxic effects are detected promptly. In general, monitoring of the following parameters is recommended: hematology at least monthly, renal function and liver function every 1 to 2 months [see Warnings and Precautions (5.1, 5.3, 5.5)].
Increase monitoring frequency during initial dosing, dose changes, or during periods of increased risk of elevated methotrexate blood levels (e.g., dehydration).
Liver Function Tests
Transient liver function test abnormalities are observed frequently after methotrexate administration and are usually not cause for modification of methotrexate therapy. Persistent liver function test abnormalities, and/or depression of serum albumin may be indicators of serious liver toxicity and require evaluation [see Warnings and Precautions (5.5)].
Pulmonary Function Tests
Pulmonary function tests may be useful if methotrexate-induced lung disease is suspected, especially if baseline measurements are available [see Warnings and Precautions (5.6)].
5.15 Risk of Improper Dosing
Both the physician and pharmacist should emphasize to the patient that the recommended dose is taken one time weekly, as directed, and that mistaken daily use of the recommended dose has led to fatal toxicity [see Dosage and Administration (2.1), Overdosage (10)].
Advise patients to measure XATMEP with an accurate milliliter measuring device. Inform patients that a household teaspoon is not an accurate measuring device and could lead to overdosage, which can result in serious adverse reactions [see Overdosage (10)]. Advise patients to ask their pharmacist to recommend an appropriate measuring device and for instructions for measuring the correct dose [see Dosage and Administration (2.1), Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling.
- •
- Bone Marrow Suppression [see Warnings and Precautions (5.1)]
- •
- Serious Infections [see Warnings and Precautions (5.2)]
- •
- Renal Toxicity and Increased Toxicity with Renal Impairment [see Warnings and Precautions (5.3)]
- •
- Gastrointestinal Toxicity [see Warnings and Precautions (5.4)]
- •
- Hepatic Toxicity [see Warnings and Precautions (5.5)]
- •
- Pulmonary Toxicity [see Warnings and Precautions (5.6)]
- •
- Hypersensitivity and Dermatologic Reactions [see Warnings and Precautions (5.7)]
- •
- Secondary Malignancies [see Warnings and Precautions (5.8)]
- •
- Ineffective Immunization and Risks Associated with Live Vaccines [see Warnings and Precautions (5.10)]
- •
- Infertility [see Warnings and Precautions (5.11)]
- •
- Increased Toxicity Due to Third‑Space Accumulation [see Warnings and Precautions (5.12)]
- •
- Soft Tissue and Bone Toxicity with Radiation Therapy [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
The most frequently reported adverse reactions include ulcerative stomatitis, leukopenia, nausea, and abdominal distress. Other frequently reported adverse reactions are malaise, fatigue, chills, fever, dizziness, and decreased resistance to infection. Folate deficiency states may increase methotrexate toxicity.
Polyarticular Juvenile Idiopathic Arthritis
The approximate incidences of adverse reactions reported in pediatric patients with JIA treated with oral, weekly doses of methotrexate (5 to 20 mg/m2/week or 0.1 to 0.65 mg/kg/week) were as follows (virtually all patients were receiving concomitant nonsteroidal anti-inflammatory drugs, and some also were taking low doses of corticosteroids): elevated liver function tests, 14%; gastrointestinal reactions (e.g., nausea, vomiting, diarrhea), 11%; stomatitis, 2%; leukopenia, 2%; headache, 1.2%; alopecia, 0.5%; dizziness, 0.2%; and rash, 0.2%. Although there is experience with dosing up to 30 mg/m2/week in JIA, the published data for doses above 20 mg/m2/week are too limited to provide reliable estimates of adverse reaction rates.
6.2 Postmarketing Experience
Additional adverse reactions which have been identified during postmarketing use of methotrexate are listed below by organ system.
Blood and Lymphatic System Disorders: Suppressed hematopoiesis causing anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, thrombocytopenia, lymphadenopathy, lymphoproliferative disorders (including reversible), hypogammaglobulinemia
Cardiovascular: Thromboembolic events (including arterial thrombosis, cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, and pulmonary embolus), pericarditis, pericardial effusion, hypotension
Eye Disorders: Optic neuropathy, transient blindness, blurred vision, ocular irritation, conjunctivitis, xerophthalmia
Gastrointestinal Disorders: Gingivitis, pharyngitis, stomatitis, anorexia, nausea, vomiting, diarrhea, hematemesis, melena, gastrointestinal ulceration and bleeding, enteritis, pancreatitis
Hepatobiliary Disorders: Hepatotoxicity, acute hepatitis, chronic fibrosis and cirrhosis, decreased serum albumin, liver enzyme elevations
Immune System Disorders: Vasculitis, lymphomas, and anaphylactoid reactions
Infections: Fatal opportunistic infections (most commonly Pneumocystis jiroveci pneumonia). There have also been reports of other infections, pneumonia, sepsis, nocardiosis, histoplasmosis, cryptococcosis, Herpes zoster, Herpes simplex hepatitis, and disseminated Herpes simplex
Metabolism: Hyperglycemia and tumor lysis syndrome
Musculoskeletal System: Stress fracture, soft tissue necrosis, osteonecrosis, arthralgia, myalgia, osteoporosis
Nervous System Disorders: Headaches, drowsiness, blurred vision, transient blindness, speech impairment (including dysarthria and aphasia), hemiparesis, paresis and convulsions have also occurred following administration of methotrexate.
Following low doses, there have been reports of transient subtle cognitive dysfunction, mood alteration, unusual cranial sensations, leukoencephalopathy, or encephalopathy.
Renal Disorders: Azotemia, hematuria, proteinuria, cystitis
Reproductive Disorders: Defective oogenesis or spermatogenesis, menstrual dysfunction, loss of libido, impotence, vaginal discharge, gynecomastia
Respiratory Disorders: Pulmonary fibrosis, respiratory failure, chronic interstitial obstructive pulmonary disease, pleuritic pain and thickening alveolitis
Skin Disorders: Erythematous rashes, pruritus, urticaria, photosensitivity, pigmentary changes, alopecia, ecchymosis, telangiectasia, acne, furunculosis, erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, skin necrosis, skin ulceration, accelerated nodulosis, and exfoliative dermatitis.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on XATMEP
Oral Antibiotics
Penicillins may reduce the renal clearance of methotrexate; increased serum concentrations of methotrexate with concomitant hematologic and gastrointestinal toxicity have been observed with methotrexate. Monitor patients accordingly [see Warnings and Precautions (5.1, 5.4)].
Trimethoprim/sulfamethoxazole has been reported to increase bone marrow suppression in patients receiving methotrexate. Monitor patients accordingly [see Warnings and Precautions (5.1)].
Hepatotoxins
The potential for increased hepatotoxicity when methotrexate is administered with other hepatotoxic agents has not been evaluated; however, hepatotoxicity has been reported in such cases. Monitor patients receiving XATMEP with other potential hepatotoxins (e.g., azathioprine, retinoids, and sulfasalazine) for possible signs of hepatotoxicity.
Probenecid
Probenecid may reduce renal elimination of methotrexate. Consider alternative drugs.
Nitrous Oxide
The use of nitrous oxide anesthesia potentiates the effect of methotrexate on folate-dependent metabolic pathways, resulting in the potential for increase toxicity. Avoid the simultaneous use of nitrous oxide and methotrexate.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), Aspirin, and Steroids
Concomitant administration of some NSAIDs with high dose methotrexate therapy has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic and gastrointestinal toxicity.
Caution should be used when NSAIDs and salicylates are administered concomitantly with lower doses of methotrexate, including XATMEP. These drugs have been reported to reduce the tubular secretion of methotrexate in an animal model and may enhance its toxicity.
Despite the potential interactions, studies of methotrexate in patients with rheumatoid arthritis, including patients with polyarticular juvenile idiopathic arthritis (pJIA), have usually included concurrent use of constant dosage regimens of NSAIDs, without apparent problems. It should be appreciated, however, that the doses used in pJIA (10 mg/m2/week as starting dose) are somewhat lower than those used in acute lymphoblastic leukemia and that larger doses could lead to unexpected toxicity. Aspirin, NSAIDs, and/or low dose steroids may be continued, although the possibility of increased toxicity with concomitant use of NSAIDs including salicylates has not been fully explored. Steroids may be reduced gradually in patients who respond to methotrexate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on published reports and methotrexate’s mechanism of action, methotrexate is a teratogen that can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman [see Data and Clinical Pharmacology (12.1)]. In pregnant women with non-malignant disease, XATMEP is contraindicated. Consider the benefits and risks of XATMEP and risks to the fetus when prescribing XATMEP to a pregnant patient with a neoplastic disease. There are no animal data that meet current standards for nonclinical developmental toxicity studies.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Published data from cases, literature reviews, and observational studies report that methotrexate exposure during pregnancy is associated with an increased risk of embryo-fetal toxicity and fetal death. Methotrexate exposure during the first trimester of pregnancy is associated with an increased incidence of spontaneous abortions and multiple adverse developmental outcomes, including skull anomalies, facial dysmorphism, central nervous system abnormalities, limb abnormalities, and sometimes cardiac anomalies and intellectual impairment. Adverse outcomes associated with exposure during second and third trimesters of pregnancy include intrauterine growth restriction and functional abnormalities. Because methotrexate is widely distributed and persists in the body for a prolonged period, there is a potential risk to the fetus from preconception methotrexate exposure.
A prospective multicenter study by U.S. and European teratology information services evaluated pregnancy outcomes in women taking methotrexate less than or equal to 30 mg/week after conception. The rate of spontaneous abortion/miscarriage in pregnant women exposed to methotrexate was 42.5% (95% confidence interval [95% CI] 29.2-58.7), which was higher than in unexposed autoimmune disease comparators (22.5%, 95% CI 16.8-29.7) and unexposed nonautoimmune disease comparators (17.3%, 95% CI 13-22.8). Of the live births, the rate of major birth defects in pregnant women exposed to methotrexate after conception was higher than in autoimmune disease comparators (adjusted odds ratio (OR) 1.8 [95% CI 0.6-5.7]) and nonautoimmune disease comparators (adjusted OR 3.1 [95% CI 1.03-9.5]). Major birth defects associated with pregnancies exposed to methotrexate after conception were not always consistent with methotrexate-associated adverse developmental outcomes.
8.2 Lactation
Risk Summary
Limited published literature report the presence of methotrexate in human milk in low amounts. The highest breast milk to plasma concentration ratio demonstrated was 0.08:1. No information is available on the effects of methotrexate on a breastfed infant or on milk production. Because of the potential for serious adverse reactions, including myelosuppression, from methotrexate in breastfed infants, advise women not to breastfeed during XATMEP therapy.
8.3 Females and Males of Reproductive Potential
Contraception
Females
XATMEP can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during and for 6 months after the final methotrexate dose.
Males
Methotrexate can cause chromosomal damage to sperm cells. Advise males with female partners of reproductive potential to use effective contraception during and for at least 3 months after the final methotrexate dose.
Infertility
Females
Based on published reports of female infertility after therapy with methotrexate, advise females of reproductive potential that XATMEP can cause impairment of fertility and menstrual dysfunction during and after cessation of therapy. It is not known if the infertility may be reversed in all affected females.
Males
Based on published reports of male infertility after therapy with methotrexate, advise males of reproductive potential that XATMEP can cause oligospermia or infertility during and after cessation of therapy. It is not known if the infertility may be reversed in all affected males.
8.4 Pediatric Use
Safety and effectiveness of XATMEP in pediatric patients have been established for the treatment of pediatric patients with acute lymphoblastic leukemia (ALL) as part of a multi-phase, combination chemotherapy maintenance regimen and for the management of pediatric patients with active polyarticular juvenile idiopathic arthritis (pJIA) [see Clinical Studies (14)].
8.6 Renal Impairment
Methotrexate elimination is reduced in patients with impaired renal function. Monitor patients with renal impairment for an extended period of time. Consider a dose reduction or, in some cases, discontinue XATMEP administration [see Warnings and Precautions (5.3)].
8.7 Hepatic Impairment
The effect of hepatic impairment on methotrexate pharmacokinetics has not been studied. Patients with hepatic impairment may be more susceptible to hepatotoxicity [see Warnings and Precautions (5.5)]. Consider dose adjustments or alternative treatments in patients with baseline hepatic impairment.
-
10 OVERDOSAGE
Manifestations
Fatal overdosage has occurred with methotrexate. Manifestations of overdosage include adverse reactions reported at pharmacologic doses, particularly hematologic and gastrointestinal reactions (e.g., leukopenia, thrombocytopenia, anemia, pancytopenia, bone marrow suppression, mucositis, stomatitis, oral ulceration, nausea, vomiting, gastrointestinal ulceration, or gastrointestinal bleeding). In some cases, no symptoms were reported.
Management
Leucovorin and levoleucovorin are indicated to diminish the toxicity and counteract the effect of inadvertently administered overdosages of methotrexate. Administer leucovorin or levoleucovorin as soon as possible after overdosage (refer to the leucovorin or levoleucovorin Prescribing Information). Monitor serum methotrexate concentrations closely to guide leucovorin or levoleucovorin therapy. Monitor serum creatinine concentrations closely because high serum methotrexate concentrations may cause renal damage leading to acute renal failure.
Glucarpidase is indicated for the treatment of toxic methotrexate concentrations in patients with delayed methotrexate clearance due to impaired renal function (refer to the glucarpidase Prescribing Information). If glucarpidase is used, do not administer leucovorin within 2 hours before or after a dose of glucarpidase because leucovorin is a substrate for glucarpidase.
In cases of massive overdosage, hydration and urinary alkalinization may be necessary to prevent the precipitation of methotrexate and/or its metabolites in the renal tubules. Neither hemodialysis nor peritoneal dialysis has been shown to improve methotrexate elimination. However, effective clearance of methotrexate has been reported with acute, intermittent hemodialysis using a high-flux dialyzer.
-
11 DESCRIPTION
XATMEP contains methotrexate, a folate analog metabolic inhibitor.
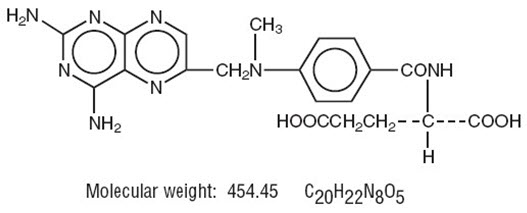
Chemically methotrexate is N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]-benzoyl]-L-glutamic acid. The structural formula is:
XATMEP is a clear yellow to orange oral solution that contains 2.5 mg of methotrexate per milliliter (equivalent to 2.74 mg of methotrexate sodium/mL). Inactive ingredients include purified water, sodium citrate, citric acid, methylparaben sodium, propylparaben sodium, and sucralose. It may also contain sodium hydroxide or hydrochloric acid for pH adjustment.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. Therefore, methotrexate interferes with DNA synthesis, repair, and cellular replication. Actively proliferating tissues such as malignant cells, bone marrow, fetal cells, buccal and intestinal mucosa, and cells of the urinary bladder are in general more sensitive to this effect of methotrexate.
The mechanism of action in pJIA is unknown; it may affect immune function.
12.2 Pharmacodynamics
Two reports describe in vitro methotrexate inhibition of DNA precursor uptake by stimulated mono-nuclear cells, and another describes in animal polyarthritis partial correction by methotrexate of spleen cell hyporesponsiveness and suppressed IL 2 production. Other laboratories, however, have been unable to demonstrate similar effects.
12.3 Pharmacokinetics
Absorption
In pediatric patients with ALL, oral absorption of methotrexate appears to be dose dependent; the absorption of doses greater than 40 mg/m2 is significantly less than that of lower doses. The extent of oral absorption ranges from 23% to 95%, and the time to peak concentration (Tmax) ranges from 0.7 hours to 4 hours after an oral dose of 15 mg/m2.
In pediatric patients with pJIA, mean serum concentrations were 0.59 micromolar (range, 0.03 to 1.40) at 1 hour, 0.44 micromolar (range, 0.01 to 1.00) at 2 hours, and 0.29 micromolar (range 0.06 to 0.58) at 3 hours following oral administration of methotrexate at a dose of 6.4 mg/m2/week to 11.2 mg/m2/week.
Effect of Food
The administration of XATMEP with food did not affect the area under the curve (AUC), but decreased the maximal concentrations (Cmax) by 50% and delayed the absorption.
Distribution
After intravenous administration, the initial volume of distribution is approximately 0.18 L/kg (18% of body weight) and steady-state volume of distribution is approximately 0.4 to 0.8 L/kg (40% to 80% of body weight).
Methotrexate competes with reduced folates for active transport across cell membranes by means of a single carrier-mediated active transport process. At serum concentrations greater than 100 micromolar, passive diffusion becomes a major pathway by which effective intracellular concentrations can be achieved.
Methotrexate in serum is approximately 50% protein bound.
Methotrexate does not penetrate the blood-cerebrospinal fluid barrier in therapeutic amounts when given orally.
Elimination
In adults, the half-life of methotrexate following administration of low dose methotrexate (less than 30 mg/m2) ranges from 3 hours to 10 hours.
In pediatric patients receiving methotrexate for ALL (6.3 mg/m2 to 30 mg/m2), the terminal half-life has been reported to range from 0.7 hours to 5.8 hours.
In pediatric patients receiving methotrexate for JIA (3.75 mg/m2 to 26.2 mg/m2), the terminal half-life has been reported to range from 0.9 hours to 2.3 hours.
Metabolism
Methotrexate undergoes hepatic and intracellular metabolism to polyglutamated forms which can be converted back to methotrexate by hydrolase enzymes. These polyglutamates act as inhibitors of dihydrofolate reductase and thymidylate synthetase. Small amounts of methotrexate polyglutamates may remain in tissues for extended periods. The retention and prolonged drug action of these active metabolites vary among different cells, tissues and tumors. A small amount of metabolism to 7‑hydroxymethotrexate may occur at doses commonly prescribed. The aqueous solubility of 7‑hydroxymethotrexate is 3- to 5-fold lower than the parent compound. Methotrexate is partially metabolized by intestinal flora after oral administration.
Excretion
Renal excretion is the primary route of elimination and is dependent upon dosage and route of administration. With IV administration, 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. There is limited biliary excretion amounting to 10% or less of the administered dose. Enterohepatic recirculation of methotrexate has been proposed.
Renal excretion occurs by glomerular filtration and active tubular secretion. Nonlinear elimination due to saturation of renal tubular reabsorption has been observed in patients at doses between 7.5 mg and 30 mg. Impaired renal function, as well as concurrent use of drugs such as weak organic acids that also undergo tubular secretion, can markedly increase methotrexate serum levels.
Methotrexate clearance decreases at higher doses. Delayed drug clearance has been identified as one of the major factors responsible for methotrexate toxicity. When a patient has delayed drug elimination due to compromised renal function, a third-space effusion, or other causes, methotrexate serum concentrations may remain elevated for prolonged periods.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Methotrexate has been evaluated in a number of animal studies for carcinogenic potential with inconclusive results. Although there is evidence that methotrexate causes chromosomal damage to animal somatic cells and human bone marrow cells, the clinical significance remains uncertain.
-
14 CLINICAL STUDIES
Polyarticular Juvenile Idiopathic Arthritis
Clinical trials in patients with polyarticular juvenile idiopathic arthritis were performed using other formulations of methotrexate.
In a 6-month, double-blind, placebo-controlled trial of 127 pediatric patients with juvenile idiopathic arthritis (JIA) (mean age, 10.1 years; age range 2.5 to 18 years, mean duration of disease, 5.1 years) on background non-steroidal anti-inflammatory drugs (NSAIDs) and/or prednisone, methotrexate given one time weekly at an oral dose of 10 mg/m2 provided significant clinical improvement compared to placebo as measured by either the physician’s global assessment, or by a patient composite (25% reduction in the articular-severity score plus improvement in parent and physician global assessments of disease activity). Over two-thirds of the patients in this trial had polyarticular-course JIA, and the numerically greatest response was seen in this subgroup treated with 10 mg/m2/week methotrexate. The overwhelming majority of the remaining patients had systemic-course JIA. All patients were unresponsive to NSAIDs; approximately one-third were using low dose corticosteroids. Weekly methotrexate at a dose of 5 mg/m2 was not significantly more effective than placebo in this trial.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
XATMEP is a clear yellow to orange oral solution that contains 2.5 mg of methotrexate per milliliter (equivalent to 2.74 mg of methotrexate sodium/mL). It is packaged in a high-density polyethylene (HDPE) bottle with a child-resistant cap and tamper-evident seal.
XATMEP is available in bottles of 60 mL (NDC 52652-2001-6) and 120 mL (NDC 52652-2001-1).
Store XATMEP refrigerated (2°C to 8°C/36°F to 46°F) tightly closed in the original container prior to dispensing.
Once dispensed, patients may store XATMEP either refrigerated (2°C to 8°C/36°F to 46°F) or at room temperature (20°C to 25°C/68°F to 77°F) with excursions permitted to 15°C to 30°C/59°F to 86°F [see USP Controlled Room Temperature]. If stored at room temperature, discard after 60 days. Avoid freezing and excessive heat.
-
17 PATIENT COUNSELING INFORMATION
Importance of Proper Dosing and Administration
Advise patients that the recommended dose should be taken one time weekly, as directed, and that mistaken daily use of the recommended dose has led to fatal toxicity [see Dosage and Administration (2.1), Warnings and Precautions (5.15)].
Advise patients and caregivers to measure XATMEP with an accurate milliliter measuring device. A household teaspoon is not an accurate measuring device. Advise patients and caregivers to ask their pharmacist to recommend an appropriate measuring device and for instructions for measuring the correct dose.
Bone Marrow Suppression and Serious Infections
Advise patients to contact their healthcare provider for new onset fever, symptoms of infection, easy bruising or persistent bleeding [see Warnings and Precautions (5.1, 5.2)].
Renal Toxicity
Advise patients that methotrexate can cause renal toxicity [see Warnings and Precautions (5.3)].
Gastrointestinal Toxicity
Advise patients to contact their healthcare provider if they develop diarrhea, vomiting, or stomatitis [see Warnings and Precautions (5.4)].
Hepatic Toxicity
Advise patients concerning the risk of hepatic toxicity and avoidance of alcohol during methotrexate treatment [see Warnings and Precautions (5.5)].
Pulmonary Toxicity
Advise patients to contact their healthcare provider for symptoms of cough, fever, and dyspnea [see Warnings and Precautions (5.6)].
Hypersensitivity Reactions
Advise patients concerning the risk for severe hypersensitivity reactions due to XATMEP treatment. These can be fatal and may include severe dermatologic reactions such as toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, and erythema multiforme. Advise patients to contact their healthcare provider for signs of a new or worsening rash [see Warnings and Precautions (5.7)].
Secondary Malignancies
Advise patients that there is a risk of secondary malignancies during or following treatment with XATMEP [see Warnings and Precautions (5.8)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.9), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during XATMEP therapy and for 6 months after the final dose [see Use in Specific Populations (8.3)].
Advise males of reproductive potential to use effective contraception during XATMEP therapy and for 3 months after the final dose [see Use in Specific Populations (8.3)].
Ineffective Immunization and Risks Associated with Live Vaccines
Advise patients to avoid receiving vaccines during treatment with XATMEP because they may not be effective and live virus vaccines may cause infection [see Warnings and Precautions (5.10)].
Infertility
Advise patients of reproductive potential that XATMEP may cause impairment of fertility, oligospermia, and menstrual dysfunction [see Warnings and Precautions (5.11), Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during therapy with XATMEP [see Use in Specific Populations (8.2)].
Proper Storage and Disposal
Advise patients to store XATMEP either refrigerated (2°C to 8°C/36°F to 46°F) or at room temperature (20°C to 25°C/68°F to 77°F) with excursions permitted to 15°C to 30°C/59°F to 86°F. If stored at room temperature, discard after 60 days. Inform patients and caregivers of the need for proper storage and disposal of dispensing bottles and dosing devices [see References (15)].
-
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Label
NDC 52652-2001-1
Xatmep®
(methotrexate)Oral Solution
2.5 mg/mLFor Oral Use Only
CAUTION: Cytotoxic agent
120 mL
azurity
pharmaceuticalsRx Only
Each 1 mL contains 2.5 mg
methotrexate (equivalent to
2.74 mg methotrexate sodium).Usual Dose:
See prescribing information.KEEP THIS AND ALL MEDICATIONS
OUT OF THE REACH OF CHILDRENStore refrigerated
2° - 8°C (36° - 46°F)
Patients may store Xatmep
either refrigerated or at
room temperature
(20° - 25°C/68° - 77°F).If stored at room temperature,
discard after 60 days.Discard on
_____/_____/_____
Manufactured for:
Azurity Pharmaceuticals, Inc.
Wilmington, MA 01887 USALot:
Exp: -
INGREDIENTS AND APPEARANCE
XATMEP
methotrexate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52652-2001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN SODIUM (UNII: CR6K9C2NHK) PROPYLPARABEN SODIUM (UNII: 625NNB0G9N) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52652-2001-1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2017 2 NDC:52652-2001-6 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208400 05/01/2017 Labeler - Azurity Pharmaceuticals, Inc. (117505635)