Label: NAFCILLIN- nafcillin sodium injection, powder, for solution
- NDC Code(s): 72603-211-01, 72603-211-10, 72603-453-01, 72603-453-10
- Packager: NorthStar Rx, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of Nafcillin for Injection and other antibacterial drugs, Nafcillin for Injection should be used only to treat ...
-
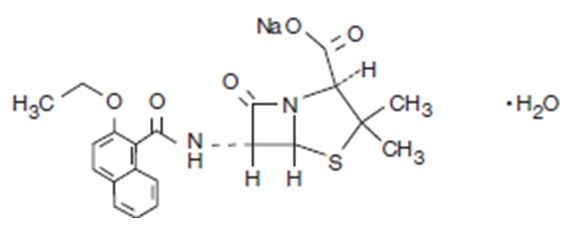
DESCRIPTIONNafcillin for Injection, USP is a semisynthetic antibiotic penicillin derived from the penicillin nucleus 6-aminopenicillanic acid. It is the sodium salt in a parenteral dosage form. The chemical ...
-
CLINICAL PHARMACOLOGYIn a study of five healthy adults administered a single 500 mg dose of nafcillin by intravenous injection over seven minutes, the mean plasma concentration of the drug was approximately 30 mcg/mL ...
-
INDICATIONS AND USAGENafcillin is indicated in the treatment of infections caused by penicillinase-producing staphylococci which have demonstrated susceptibility to the drug. Culture and susceptibility tests should be ...
-
CONTRAINDICATIONSA history of a hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
-
WARNINGSSerious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more likely to occur in individuals with a ...
-
PRECAUTIONSGeneral - Nafcillin should generally not be administered to patients with a history of sensitivity to any penicillin. Penicillin should be used with caution in individuals with histories of ...
-
ADVERSE REACTIONSBody as a Whole - The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent (see WARNINGS). Sensitization is usually the result of treatment, but some individuals ...
-
OVERDOSAGENeurotoxic reactions similar to those observed with penicillin G may arise with intravenous doses of nafcillin especially in patients with concomitant hepatic insufficiency and renal dysfunction ...
-
DOSAGE AND ADMINISTRATIONNafcillin for Injection is available for intramuscular and intravenous use. The usual IV dosage for adults is 500 mg every 4 hours. For severe infections, 1 gram every 4 hours is recommended ...
-
HOW SUPPLIEDNafcillin for Injection, USP contains nafcillin sodium equivalent to 1 gram or 2 grams of nafcillin and is supplied as follows: NDC 72603-211-10 1 gram vial Carton of 10 - NDC ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 gram per vial - Container LabelRx only - NDC 72603-453-01 - Nafcillin for Injection, USP - 1 gram per vial - Buffered - for IV or IM use - NorthStar
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2 gram per vial - Container LabelRx only - NDC 72603-453-01 - Nafcillin for Injection, USP - 1 gram per vial - Buffered - for IV or IM use - NorthStar
-
INGREDIENTS AND APPEARANCEProduct Information