Label: LIDOCAINE HYDROCHLORIDE solution
- NDC Code(s): 72888-125-26
- Packager: Advagen Pharma Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONA Topical Anesthetic for the Mucous Membranes of the Mouth and Pharynx. For Oral Use Only
-
BOXED WARNING
(What is this?)
WARNING: Life-threatening and fatal events in infants and young children
Postmarketing cases of seizures, cardiopulmonary arrest, and death in patients under the age of 3 years have been reported with use of Lidocaine Hydrochloride Oral Topical Solution 2% (Viscous) when it was not administered in strict adherence to the dosing and administration recommendations. In the setting of teething pain, Lidocaine Hydrochloride Oral Topical Solution 2% (Viscous) should generally not be used. For other conditions, the use of the product in patients less than 3 years of age should be limited to those situations where safer alternatives are not available or have been tried but failed.
To decrease the risk of serious adverse events with use of Lidocaine Hydrochloride Oral Topical Solution 2% (Viscous), instruct caregivers to strictly adhere to the prescribed dose and frequency of administration and store the prescription bottle safely out of reach of children.
Close -
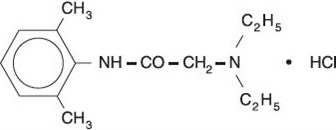
DESCRIPTIONLidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous) contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous ...
-
COMPOSITION OF SOLUTIONEach mL contains 20 mg of lidocaine hydrochloride and following inactive ingredients: banana flavor NAT WONF, carboxymethylcellulose sodium, methylparaben, propylparaben, saccharin sodium and ...
-
CLINICAL PHARMACOLOGYMechanism of Action: Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic ...
-
INDICATIONS AND USAGELidocaine Hydrochloride Oral Topical Solution 2% (Viscous) is indicated for the production of topical anesthesia of irritated or inflamed mucous membranes of the mouth and pharynx. It is also ...
-
CONTRAINDICATIONSLidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type, or to other components of the solution.
-
WARNINGSEXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ...
-
PRECAUTIONSInformation for Patients: Parents and caregivers should be cautioned about the following: For patients under 3 years of age, special care must be given to accurately measuring the prescribed ...
-
ADVERSE REACTIONSAdverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general ...
-
Central Nervous System:
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double ...
-
Cardiovascular System:
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
-
Allergic:
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent ...
-
OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics (see - ADVERSE REACTIONS, WARNINGS, and ...
-
DOSAGE AND ADMINISTRATIONAdult: The maximum recommended single dose of Lidocaine Hydrochloride Oral Topical Solution 2% (Viscous) for healthy adults should be such that the dose of lidocaine does not exceed 4.5 mg/kg or ...
-
HOW SUPPLIEDLidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous) is a clear, colorless , viscous solution supplied in 100 mL low density polyethylene squeeze bottles. Supplied with press-in bottle ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Advagen Pharma Ltd., East Windsor, NJ 08520, USA - Revised: 00, 10/2024
-
INSTRUCTIONS FOR USELidocaine Hydrochloride Oral Topical Solution, USP - Each ml contains 2% Lidocaine - Instructions for Use - Read these instructions carefully to learn how to use the medicine - dispensing ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANELLidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% - NDC 72888-125-26 -100 mL Bottle Label - Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% - NDC 72888-125-26 ...
-
INGREDIENTS AND APPEARANCEProduct Information