Label: TEPYLUTE- thiotepa injection
- NDC Code(s): 81927-105-01, 81927-106-01
- Packager: Shorla Oncology Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 4, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TEPYLUTE safely and effectively. See full prescribing information for TEPYLUTE. TEPYLUTE (thiotepa) injection, for intravenous ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SEVERE MYELOSUPPRESSION and CARCINOGENICITY
- TEPYLUTE may cause severe marrow suppression, and high doses may cause marrow ablation with resulting infection or bleeding. Monitor hematologic laboratory parameters. [see Warnings and Precautions (5.1)].
- TEPYLUTE should be considered potentially carcinogenic in humans [see Warnings and Precautions (5.7)].
-
1 INDICATIONS AND USAGE1.1 Adenocarcinoma of the Breast or Ovary - TEPYLUTE is indicated for treatment of adenocarcinoma of the breast or ovary.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Adenocarcinoma of the Breast or Ovary - The recommended dose of TEPYLUTE for treatment of adenocarcinoma of the breast or ovary is 0.3 mg/kg to 0.4 mg/kg intravenously ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 15 mg/1.5 mL (10 mg/mL) of thiotepa in a clear, colorless or almost colorless solution in single-dose vial. Injection: 100 mg/10 mL (10 mg/mL) of thiotepa in a clear, colorless or ...
-
4 CONTRAINDICATIONSTEPYLUTE is contraindicated in: Patients with severe hypersensitivity to thiotepa [see Warnings and Precautions (5.2)] Concomitant use with live or attenuated vaccines [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - For patients receiving TEPYLUTE for treatment of adenocarcinoma of the breast or adenocarcinoma of the ovary, if the bone marrow has been compromised by prior irradiation ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in other sections of the label: Myelosuppression [see Warnings and Precautions (5.1)] Infection [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Effect of Cytochrome CYP3A Inhibitors and Inducers - In vitro studies suggest that thiotepa is metabolized by CYP3A4 and CYP2B6 to its active metabolite TEPA. Avoid coadministration of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - TEPYLUTE can cause fetal harm when administered to a pregnant woman based on findings from animals and the drug’s mechanism of action [see Clinical Pharmacology ...
-
10 OVERDOSAGEThere is no experience with overdoses of thiotepa. The most important adverse reactions expected in case of overdose are myeloablation and pancytopenia [see Nonclinical Toxicology (13.1)]. There ...
-
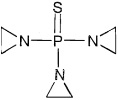
11 DESCRIPTIONTEPYLUTE injection contains thiotepa, an alkylating drug. The chemical name for thiotepa is Tris(1-aziridinyl)phosphine sulfide. Thiotepa has the following structural formula: Thiotepa has the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Thiotepa is an alkylating drug of the polyfunctional type, related chemically and pharmacologically to the nitrogen mustard. The radiomimetic action of thiotepa is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In mice, repeated intraperitoneal (IP) administration of thiotepa (1.15 or 2.3 mg/kg three times per week for 52 or 43 weeks ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. [Accessed from http://www.osha.gov/SLTC/hazardousdrugs/index.html].
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - TEPYLUTE injection is supplied as a clear, colorless or almost colorless solution. The vial stopper is not made with natural rubber latex. TEPYLUTE Injection - NDC ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity - Counsel patients on the signs and symptoms of hypersensitivity and to seek immediate emergency assistance if they develop any of these signs and symptoms [see Warnings and ...
-
Principal Display Panel - 15 mg/1.5 mL Vial LabelNDC 81927-105-01 - Rx Only - Tepylute - (thiotepa) injection - 15 mg/1.5 mL - (10 mg/mL) For intravenous infusion. MUST BE DILUTED PRIOR TO USE. Single-dose vial - Discard unused portion
-
Principal Display Panel - 15 mg/1.5 mL CartonNDC 81927-105-01 - Rx Only - Tepylute - (thiotepa) injection - 15 mg/1.5 mL - (10 mg/mL) For Intravenous Infusion. MUST BE DILUTED PRIOR TO USE. WARNING: Hazardous Drug - Contains one single-dose ...
-
Principal Display Panel - 100 mg/10 mL Vial LabelNDC 81927-106-01 - Rx Only - Tepylute - (thiotepa) injection - 100 mg/10 mL - (10 mg/mL) For intravenous infusion. MUST BE DILUTED PRIOR TO USE. Multiple-Dose vial
-
Principal Display Panel - 100 mg/10 mL CartonNDC 81927-106-01 - Rx Only - Tepylute - (thiotepa) injection - 100 mg/10 mL - (10 mg/mL) For Intravenous Infusion. MUST BE DILUTED PRIOR TO USE. WARNING: Hazardous Drug - Contains one Multiple-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information