Label: VENTOLIN HFA- albuterol sulfate aerosol, metered
- NDC Code(s): 70518-1081-0
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0173-0682
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VENTOLIN HFA safely and effectively. See full prescribing information for VENTOLIN HFA. VENTOLIN HFA (albuterol sulfate inhalation ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Bronchospasm - VENTOLIN HFA is indicated for the treatment or prevention of bronchospasm in adult and pediatric patients aged 4 years and older with reversible obstructive airway ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Bronchospasm (Acute Episodes or Symptoms Associated with Bronchospasm) Adult and pediatric patients aged 4 years and older: 2 inhalations by oral inhalation repeated ...
-
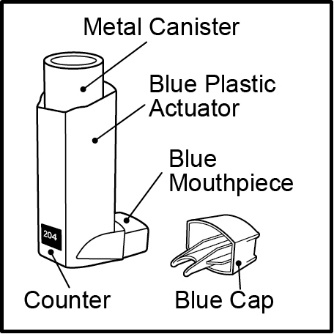
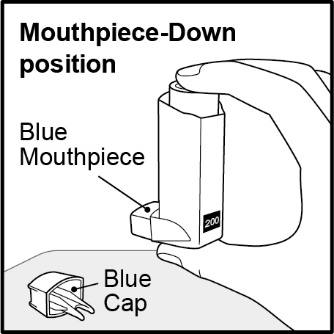
3 DOSAGE FORMS AND STRENGTHSInhalation aerosol: 108 mcg of albuterol sulfate (90 mcg of albuterol base) from the mouthpiece per actuation. Blue plastic inhaler with a blue cap containing a pressurized metered-dose aerosol ...
-
4 CONTRAINDICATIONSVENTOLIN HFA is contraindicated in patients with a history of hypersensitivity to any of the ingredients - [see Warnings and Precautions ( 5.6), Description ( 11)] .
-
5 WARNINGS AND PRECAUTIONS5.1 Paradoxical Bronchospasm - VENTOLIN HFA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with VENTOLIN HFA, it should ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Paradoxical bronchospasm - [see Warnings and Precautions ( 5.1)] Cardiovascular ...
-
7 DRUG INTERACTIONSOther short-acting sympathomimetic aerosol bronchodilators should not be used concomitantly with albuterol. If additional adrenergic drugs are to be administered by any route, they should be used ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asthma medications during pregnancy. For more ...
-
10 OVERDOSAGEThe expected signs and symptoms with overdosage of albuterol are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of ...
-
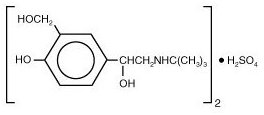
11 DESCRIPTIONThe active component of VENTOLIN HFA is albuterol sulfate, USP, the racemic form of albuterol and a relatively selective beta - 2-adrenergic bronchodilator. Albuterol sulfate has the chemical ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta - 2-adrenergic receptors compared with ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year study in Sprague-Dawley rats, albuterol sulfate caused a dose-related increase in the incidence of benign leiomyomas of the ...
-
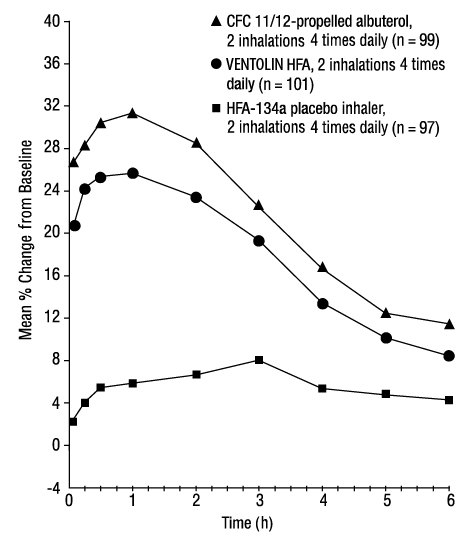
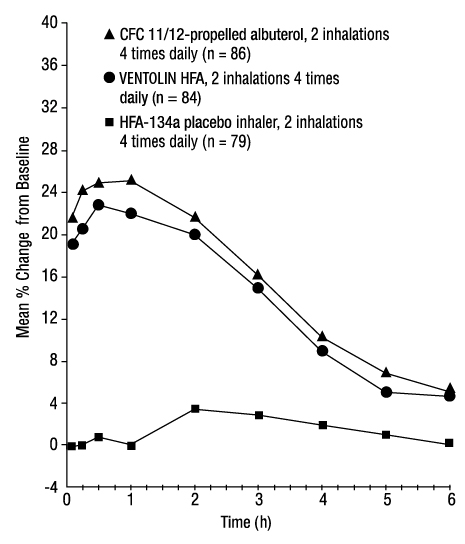
14 CLINICAL STUDIES14.1 Bronchospasm Associated with Asthma - Adult and Adolescent Subjects Aged 12 Years and Older - The efficacy of VENTOLIN HFA was evaluated in two 12-week, randomized, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVENTOLIN HFA is supplied in the following boxes of 1 as a pressurized aluminum canister fitted with a counter and supplied with a blue plastic actuator with a blue cap: NDC ...
-
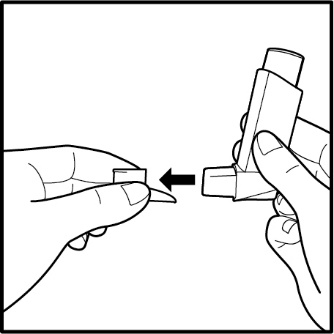
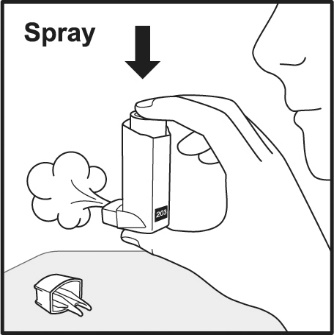
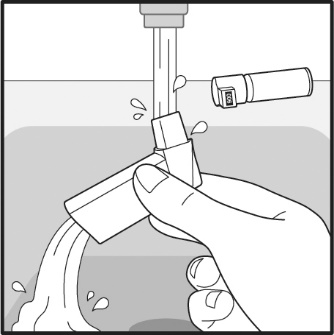
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Frequency of Use - Inform patients that the action of VENTOLIN HFA should last up to 4 ...
-
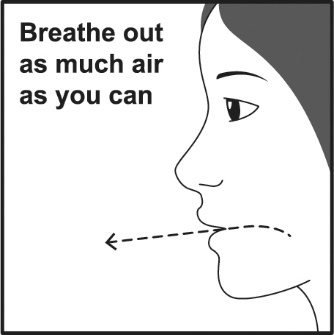
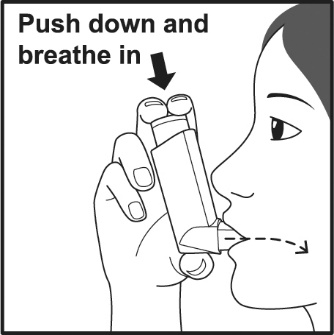
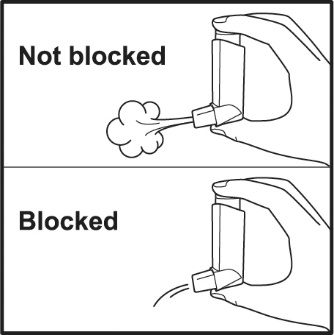
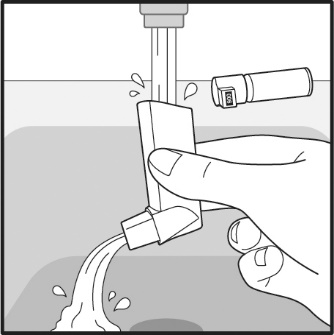
PATIENT PACKAGE INSERTPATIENT INFORMATION - VENTOLIN (VENT o lin) HFA - (albuterol sulfate inhalation aerosol) for oral inhalation use - What is VENTOLIN HFA? VENTOLIN HFA is a prescription inhaled ...
-
PRINCIPAL DISPLAY PANELDRUG: VENTOLINHFA HFA - GENERIC: albuterol sulfate - DOSAGE: AEROSOL, METERED - ADMINSTRATION: RESPIRATORY (INHALATION) NDC: 70518-1081-0 - PACKAGING: 200 in 1 INHALER - ACTIVE INGREDIENT(S): ALBUTEROL ...
-
INGREDIENTS AND APPEARANCEProduct Information