Label: METHOCARBAMOL tablet
- NDC Code(s): 72789-429-20, 72789-429-30, 72789-429-40, 72789-429-60
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 69584-612
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
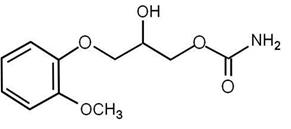
DESCRIPTIONMethocarbamol Tablets, USP, 500 mg and 750 mg, a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties. The ...
-
CLINICAL PHARMACOLOGYThe mechanism of action of methocarbamol in humans has not been established, but may be due to general central nervous system (CNS) depression. It has no direct action on the contractile mechanism ...
-
INDICATIONS AND USAGEMethocarbamol is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of ...
-
CONTRAINDICATIONSMethocarbamol is contraindicated in patients hypersensitive to methocarbamol or to any of the tablet components.
-
WARNINGSSince methocarbamol may possess a general CNS depressant effect, patients receiving methocarbamol tablets should be cautioned about combined effects with alcohol and other CNS depressants. Safe ...
-
PRECAUTIONSInformation for Patients - Patients should be cautioned that methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery. Because ...
-
ADVERSE REACTIONSAdverse reactions reported coincident with the administration of methocarbamol include: Body as a whole:Anaphylactic reaction, angioneurotic edema, fever, headache - Cardiovascular ...
-
OVERDOSAGELimited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following ...
-
DOSAGE AND ADMINISTRATIONMethocarbamol, 500 mg — Adults: Initial dosage: 3 tablets q.i.d. Maintenance dosage: 2 tablets q.i.d. Methocarbamol, 750 mg — Adults: Initial dosage: 2 tablets q.i.d. Maintenance ...
-
HOW SUPPLIEDMethocarbamol Tablets, USP 750 mg — white, capsule shape, convex face, debossed “612” on one side and debossed “O” on the reverse side. Available in: bottles of 20, NDC 72789-429-20 - bottles ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELMETHOCARBAMOL - TABLETS, USP - 750 mg - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information