Label: OFLOXACIN solution/ drops
- NDC Code(s): 24208-434-05, 24208-434-10
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION(Sterile) Rx only
-
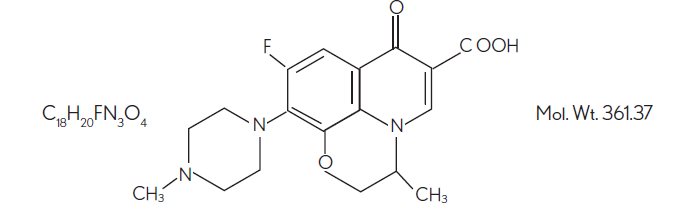
DESCRIPTIONOfloxacin ophthalmic solution, 0.3% is a sterile ophthalmic solution. It is a fluorinated carboxyquinolone anti-infective for topical ophthalmic use. Chemical ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - Serum, urine and tear concentrations of ofloxacin were measured in 30 healthy women at various time points during a ten-day course of treatment with ofloxacin ophthalmic ...
-
INDICATIONS AND USAGEOfloxacin ophthalmic solution is indicated for the treatment of infections caused by susceptible strains of the following bacteria in the conditions listed below: CONJUNCTIVITIS: Gram-positive ...
-
CONTRAINDICATIONSOfloxacin ophthalmic solution is contraindicated in patients with a history of hypersensitivity to ofloxacin, to other quinolones, or to any of the components in this medication (see ...
-
WARNINGSNOT FOR INJECTION. Ofloxacin ophthalmic solution should not be injected subconjunctivally, nor should it be introduced directly into the anterior chamber of the eye. There are rare reports of ...
-
PRECAUTIONSGeneral - As with other anti-infectives, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs discontinue use and institute alternative ...
-
ADVERSE REACTIONSOphthalmic Use - The most frequently reported drug-related adverse reaction was transient ocular burning or discomfort. Other reported reactions include stinging, redness, itching, chemical ...
-
DOSAGE AND ADMINISTRATIONThe recommended dosage regimen for the treatment of bacterial conjunctivitis is: Days 1 and 2 Instill one to two drops every two ...
-
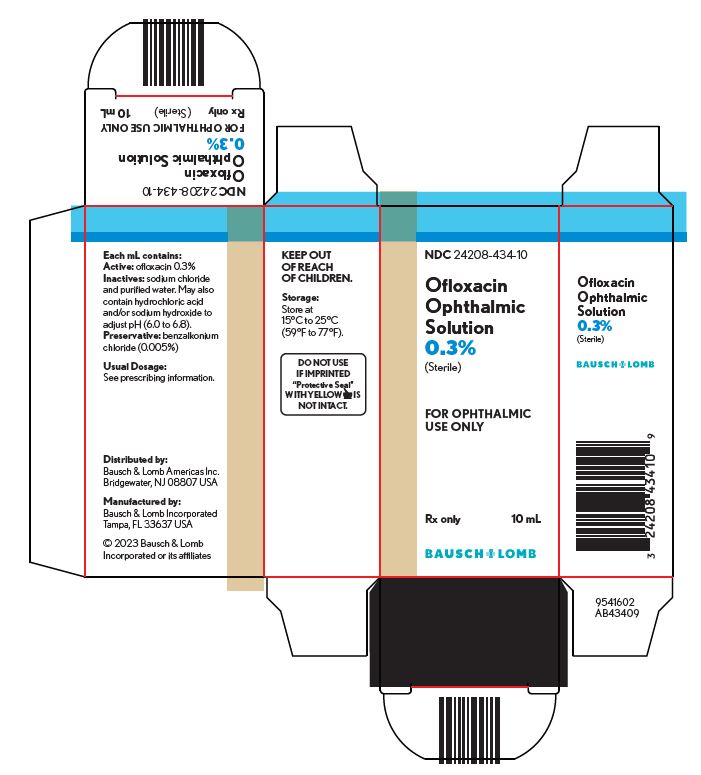
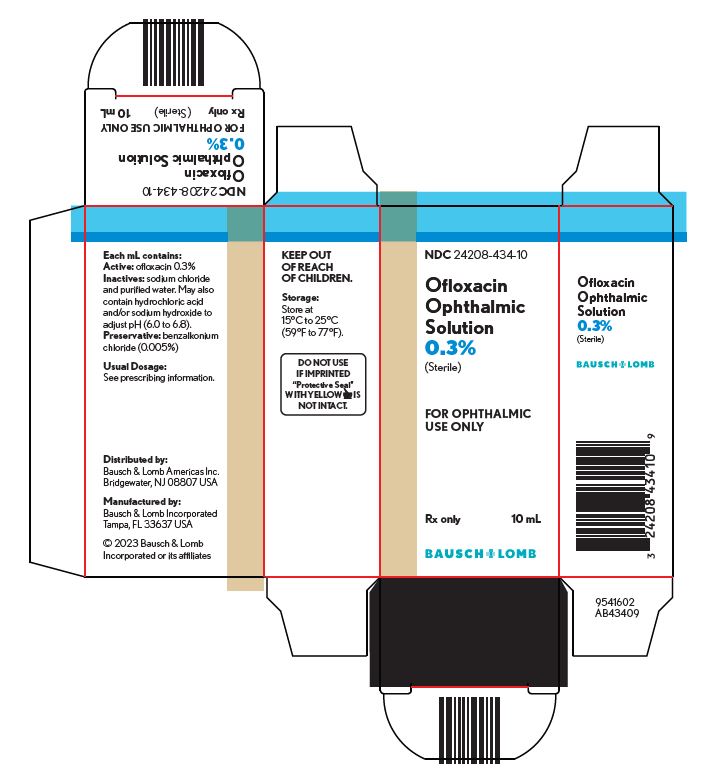
HOW SUPPLIEDOfloxacin ophthalmic solution, 0.3% is supplied sterile in plastic dropper bottles with tan caps in the following sizes: 5 mL NDC 24208-434-05 - 10 mL NDC 24208-434-10
-
STORAGE AND HANDLINGNOTE: Store at 15°C to 25°C (59°F to 77°F). KEEP OUT OF REACH OF CHILDREN. FOR OPHTHALMIC USE ONLY. Distributed by: Bausch & Lomb Americas Inc. Bridgewater, NJ 08807 USA - Manufactured ...
-
Package/Label Display Panel NDC 24208-434-10 - Ofloxacin - Ophthalmic - Solution - 0.3% Sterile - FOR OPHTHALMIC - USE ONLY - Rx only - 10 mL - BAUSCH + LOMB - 9541602 - AB43409
-
INGREDIENTS AND APPEARANCEProduct Information