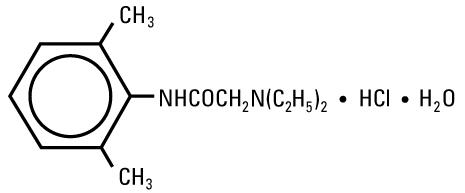Label: LIDOCAINE HYDROCHLORIDE injection, solution
- NDC Code(s): 0409-1323-05, 0409-1323-15, 0409-4903-11, 0409-4903-34, view more
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONAQUEOUS SOLUTIONS FOR - ACUTE MANAGEMENT OF - VENTRICULAR ARRHYTHMIAS - Ansyr® Plastic Syringe - LifeShield® Abboject® Syringe - Rx only
-
DESCRIPTIONLidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of an antiarrhythmic agent administered intravenously by either direct injection or continuous infusion. It is available ...
-
CLINICAL PHARMACOLOGYMechanism of Action and Electrophysiology: Studies of the effects of therapeutic concentrations of lidocaine on the electrophysiological properties of mammalian Purkinje fibers have shown that ...
-
INDICATIONS AND USAGELidocaine hydrochloride injection administered intravenously or intramuscularly, is specifically indicated in the acute management of ventricular arrhythmias such as those occurring in relation to ...
-
CONTRAINDICATIONSLidocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type. Lidocaine hydrochloride should not be used in patients with ...
-
WARNINGSIN ORDER TO MANAGE POSSIBLE ADVERSE REACTIONS, RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHEN LIDOCAINE HYDROCHLORIDE INJECTION IS USED ...
-
PRECAUTIONS1. General: Caution should be employed in the use of lidocaine hydrochloride in patients with severe liver or kidney disease because accumulation of the drug or metabolites may occur ...
-
ADVERSE REACTIONSAdverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. Adverse experiences may result from high plasma ...
-
DRUG ABUSE AND DEPENDENCEAlthough specific studies have not been conducted, lidocaine HCl has been used clinically without evidence of abuse of this drug or of psychological or physical dependence as a result of its ...
-
OVERDOSAGEOverdosage of lidocaine HCl usually results in signs of central nervous system or cardiovascular toxicity (see ADVERSE REACTIONS). Should convulsions or signs of respiratory depression and ...
-
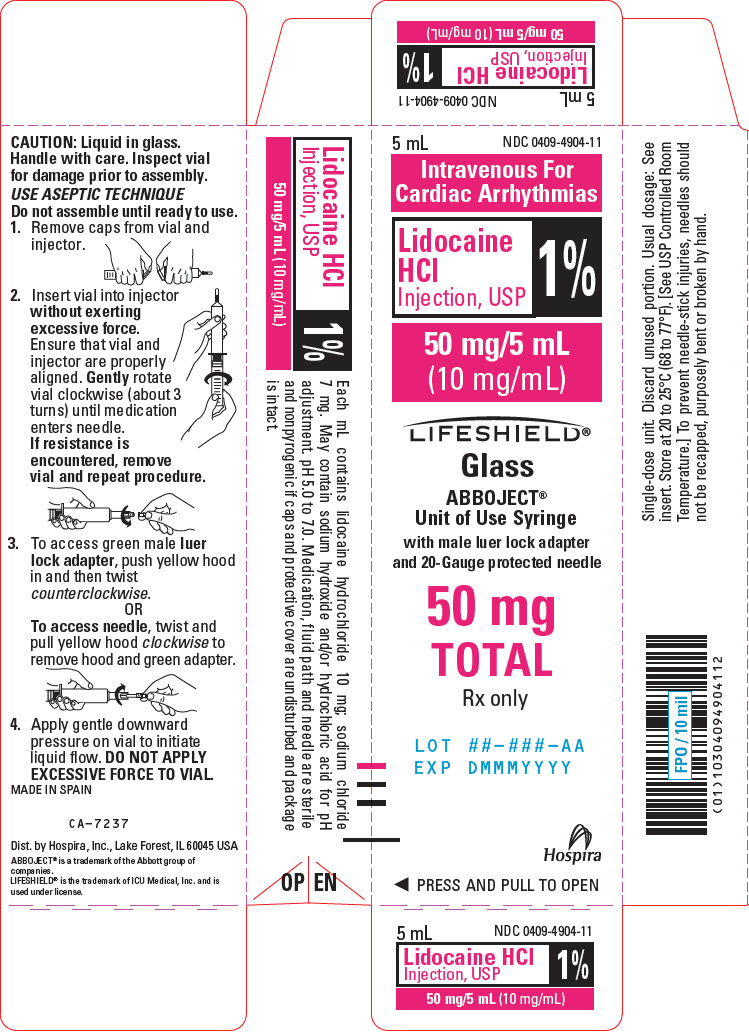
DOSAGE AND ADMINISTRATIONAdults: Single Direct Intravenous Injection (bolus): ONLY THE 5 mL, 50 MG or 100 MG DOSAGE SIZES should be used for direct intravenous injection. The usual dose is 50 to 100 mg of lidocaine ...
-
HOW SUPPLIEDLidocaine Hydrochloride Injection, USP is supplied as follows: NDC No. Container - Concentration - Size - Total (mg) **Abboject® Unit of Use Syringe with Male Luer Lock Adapter ...
-
SPL UNCLASSIFIED SECTIONLIFESHIELD® is the trademark of ICU Medical, Inc. and is used under license. LAB-1021-3.0 - Revised: 04/2018
-
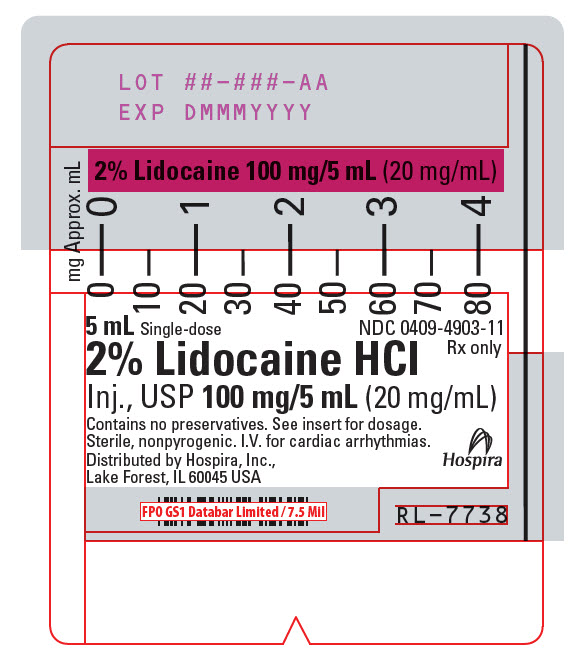
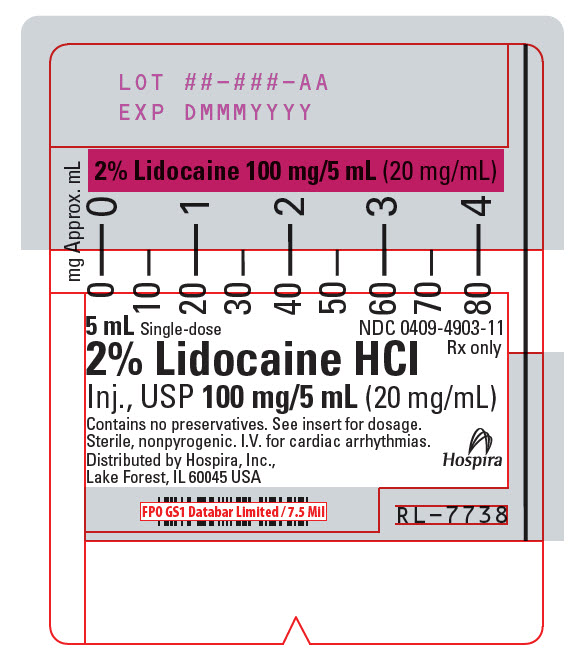
PRINCIPAL DISPLAY PANEL - 20 mg/mL Syringe Label5 mL Single-dose - NDC 0409-4903-11 - Rx only - 2% Lidocaine HCl - Inj., USP 100 mg/5 mL (20 mg/mL) Contains no preservatives. See insert for dosage. Sterile, nonpyrogenic. I.V. for cardiac ...
-
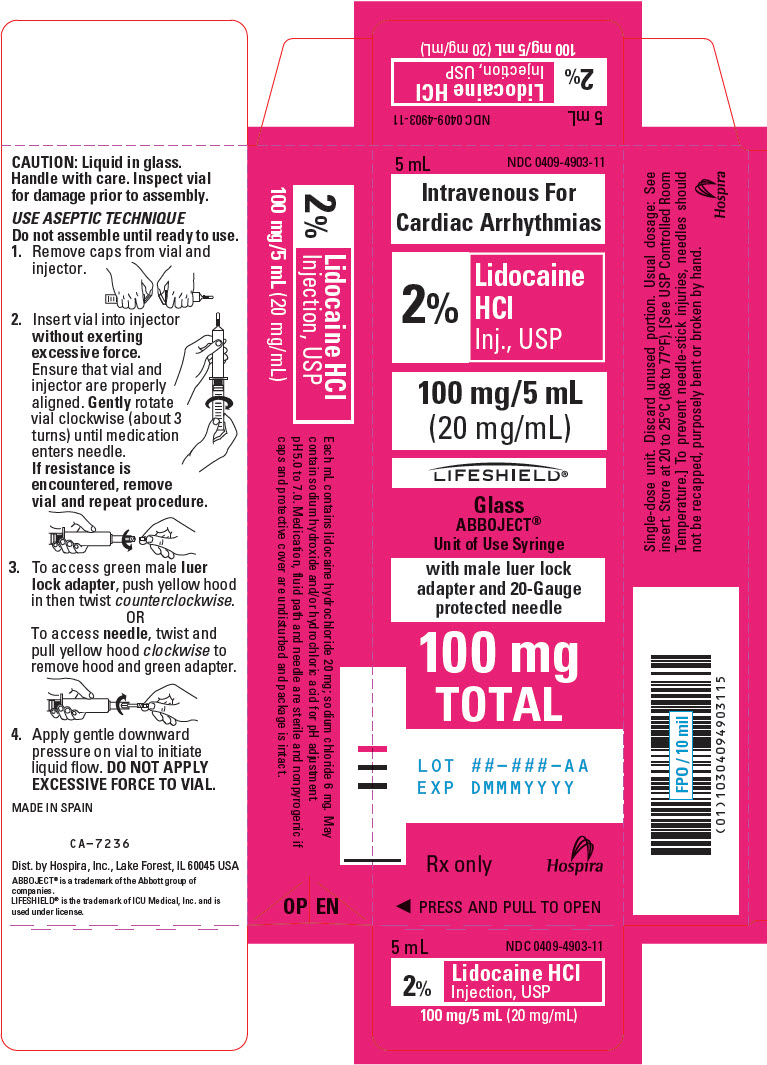
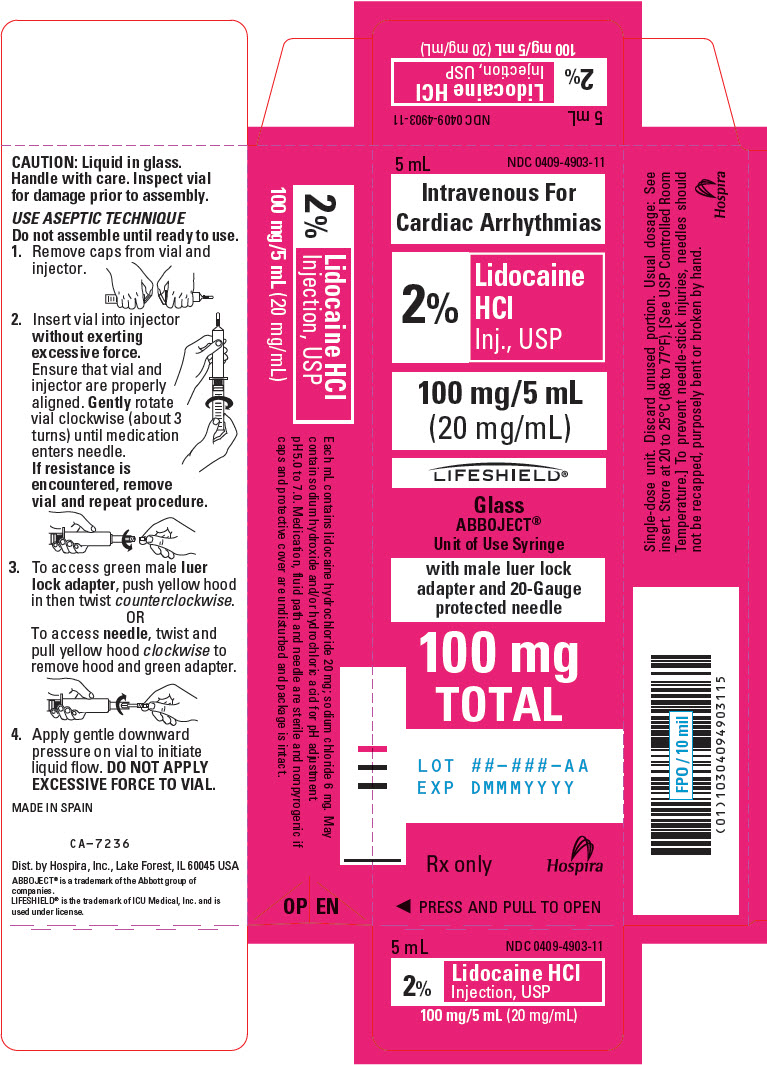
PRINCIPAL DISPLAY PANEL - 20 mg/mL Syringe Carton - LIFESHIELD5 mL - NDC 0409-4903-11 - Intravenous For - Cardiac Arrhythmias - 2% Lidocaine - HCl - Inj., USP - 100 mg/5 mL - (20 mg/mL) LIFESHIELD® Glass - ABBOJECT® Unit of Use Syringe - with male luer lock - adapter and ...
-
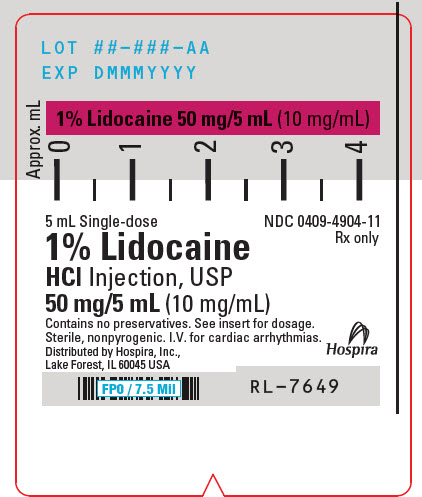
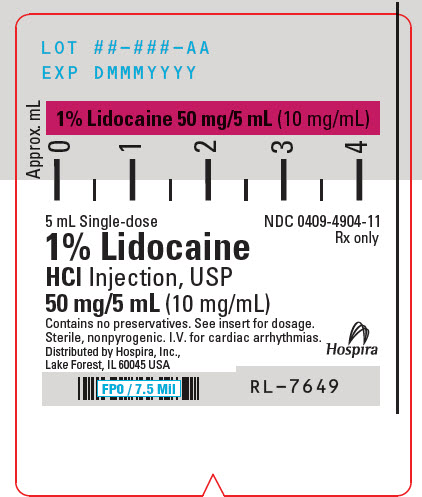
PRINCIPAL DISPLAY PANEL - 10 mg/mL Syringe Label - LIFESHIELD5 mL Single-dose - NDC 0409-4904-11 - Rx only - 1% Lidocaine - HCl Injection, USP - 50 mg/5 mL (10 mg/mL) Contains no preservatives. See insert for dosage. Sterile, nonpyrogenic. I.V. for cardiac ...
-
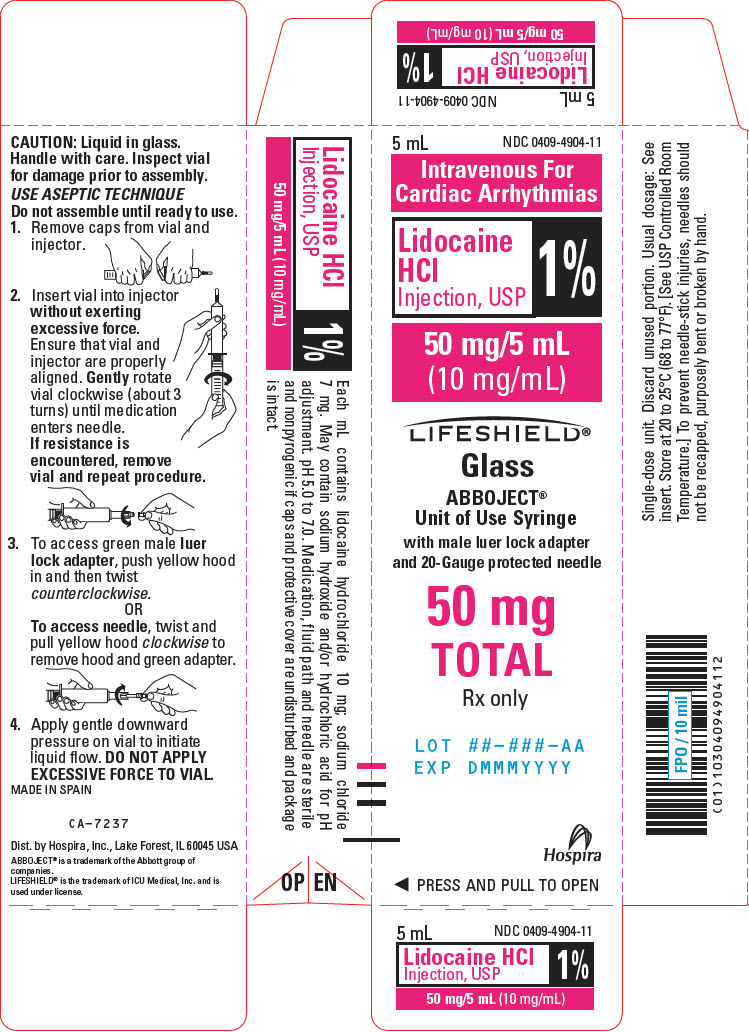
PRINCIPAL DISPLAY PANEL - 10 mg/mL Syringe Carton - LIFESHIELD5 mL - NDC 0409-4904-11 - Intravenous For - Cardiac Arrhythmias - Lidocaine - HCl - Injection, USP - 1% 50 mg/5 mL - (10 mg/mL) LIFESHIELD® Glass - ABBOJECT® Unit of Use Syringe - with male luer lock adapter ...
-
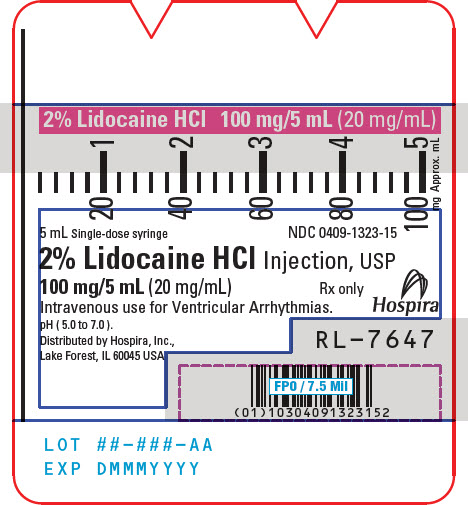
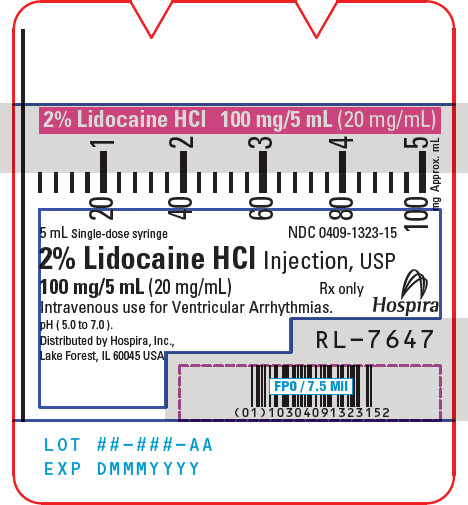
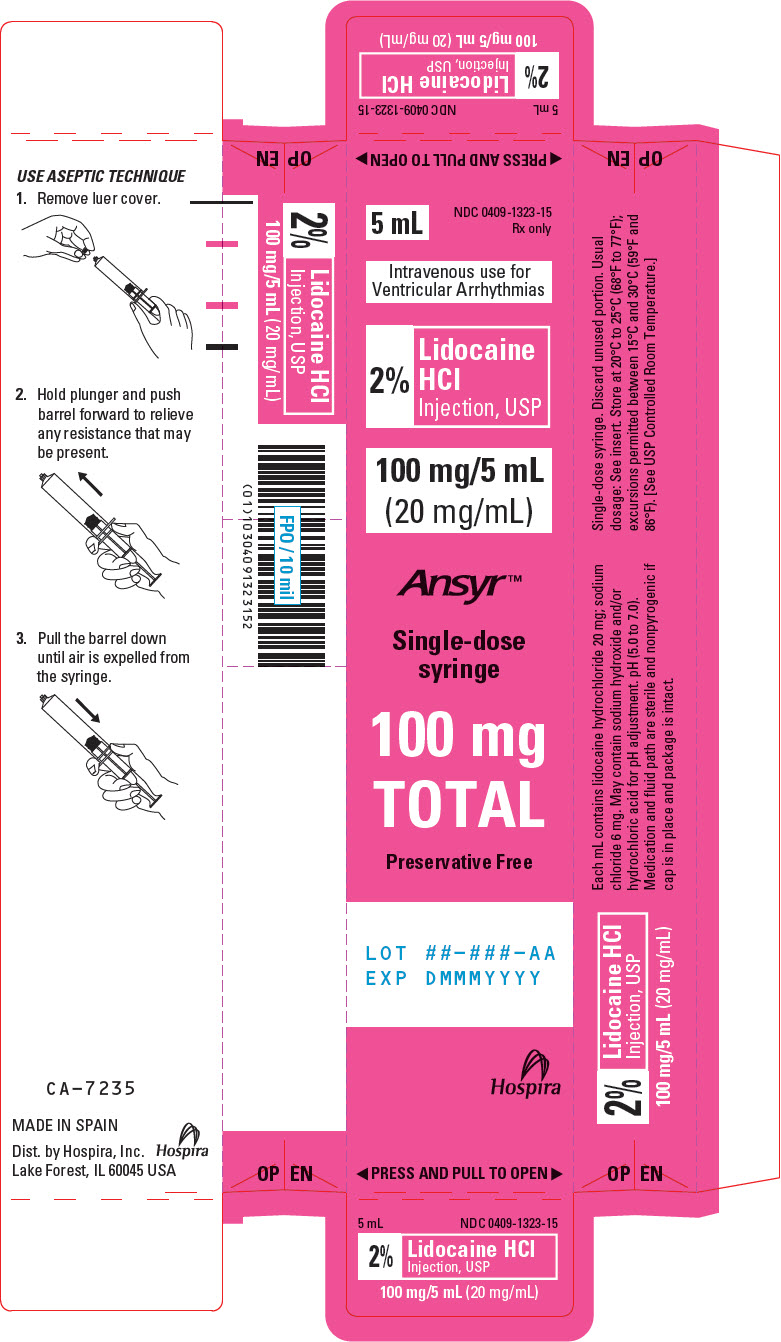
PRINCIPAL DISPLAY PANEL - 20 mg/mL Syringe Label5 mL Single-dose syringe - NDC 0409-1323-15 - 2% Lidocaine HCl Injection, USP - 100 mg/5 mL (20 mg/mL) Rx only - Intravenous use for Ventricular Arrhythmias. pH ( 5.0 to 7.0 ). Hospira - Distributed by ...
-
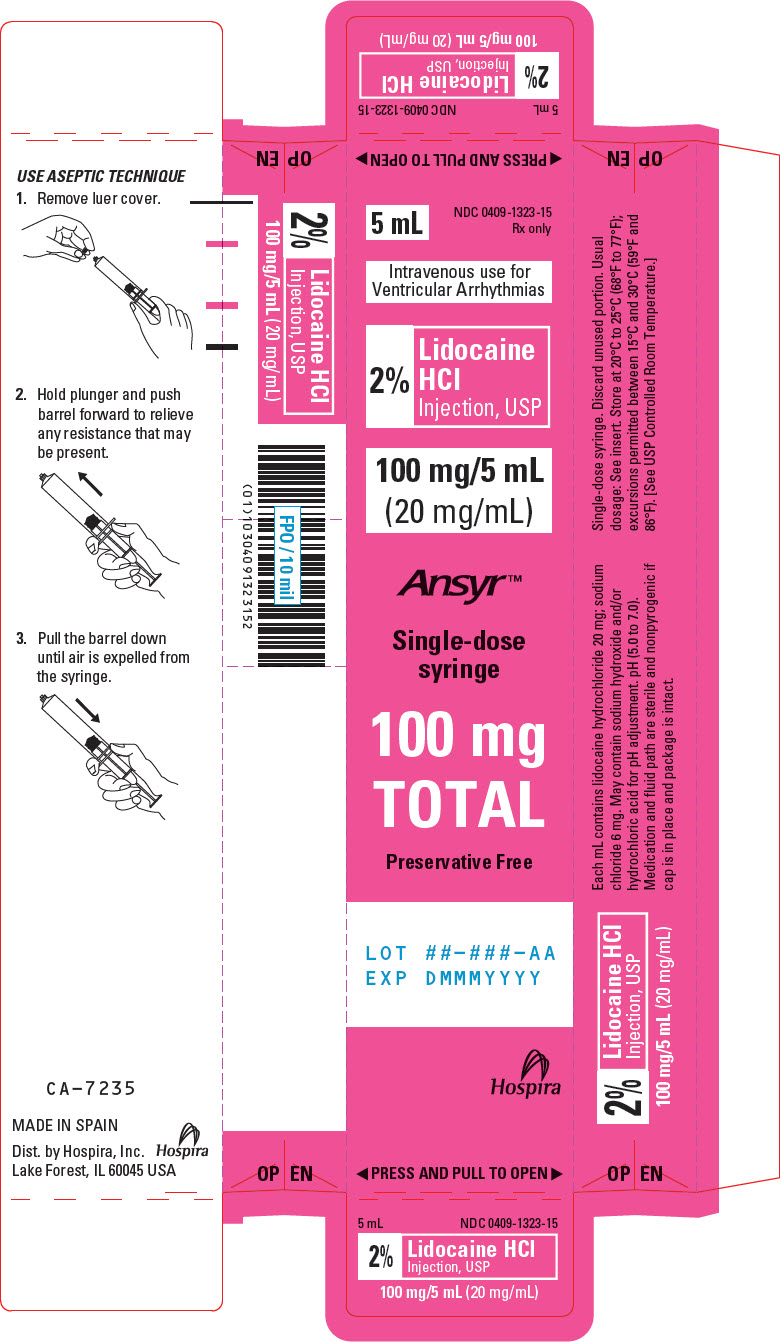
PRINCIPAL DISPLAY PANEL - 20 mg/mL Syringe Carton5 mL - NDC 0409-1323-15 - Rx only - Intravenous use for - Ventricular Arrhythmias - 2% Lidocaine - HCl - Injection, USP - 100 mg/5 mL - (20 mg/mL) Ansyr™ Single-dose - syringe - 100 mg - TOTAL - Preservative ...
-
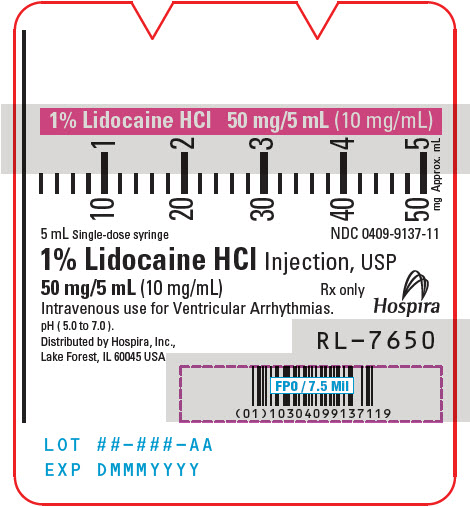
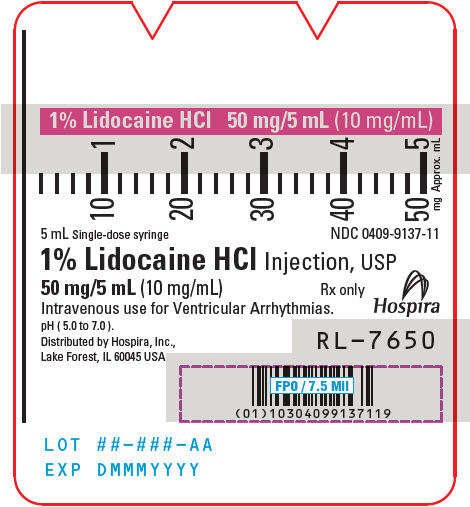
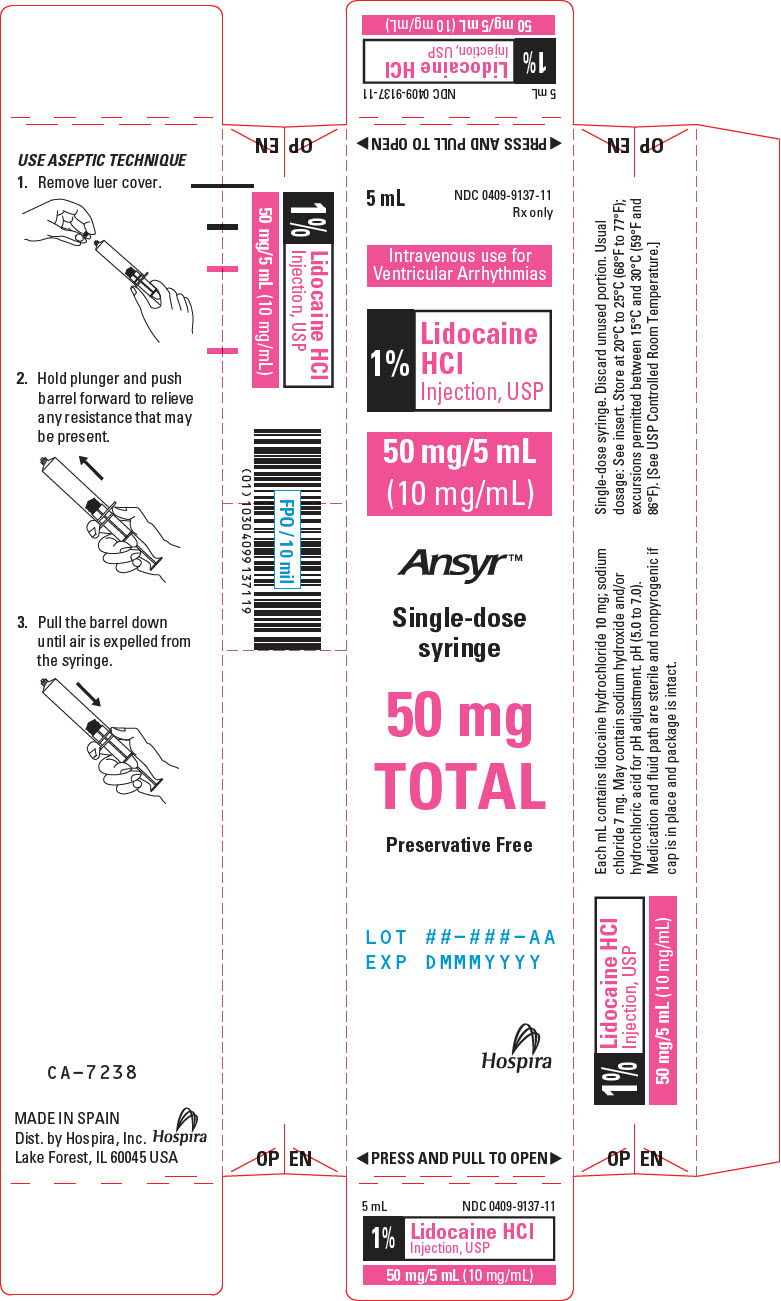
PRINCIPAL DISPLAY PANEL - 10 mg/mL Syringe Label5 mL Single-dose syringe - NDC 0409-9137-11 - 1% Lidocaine HCl Injection, USP - 50 mg/5 mL (10 mg/mL) Rx only - Intravenous use for Ventricular Arrhythmias. pH ( 5.0 to 7.0 ). Hospira - Distributed by ...
-
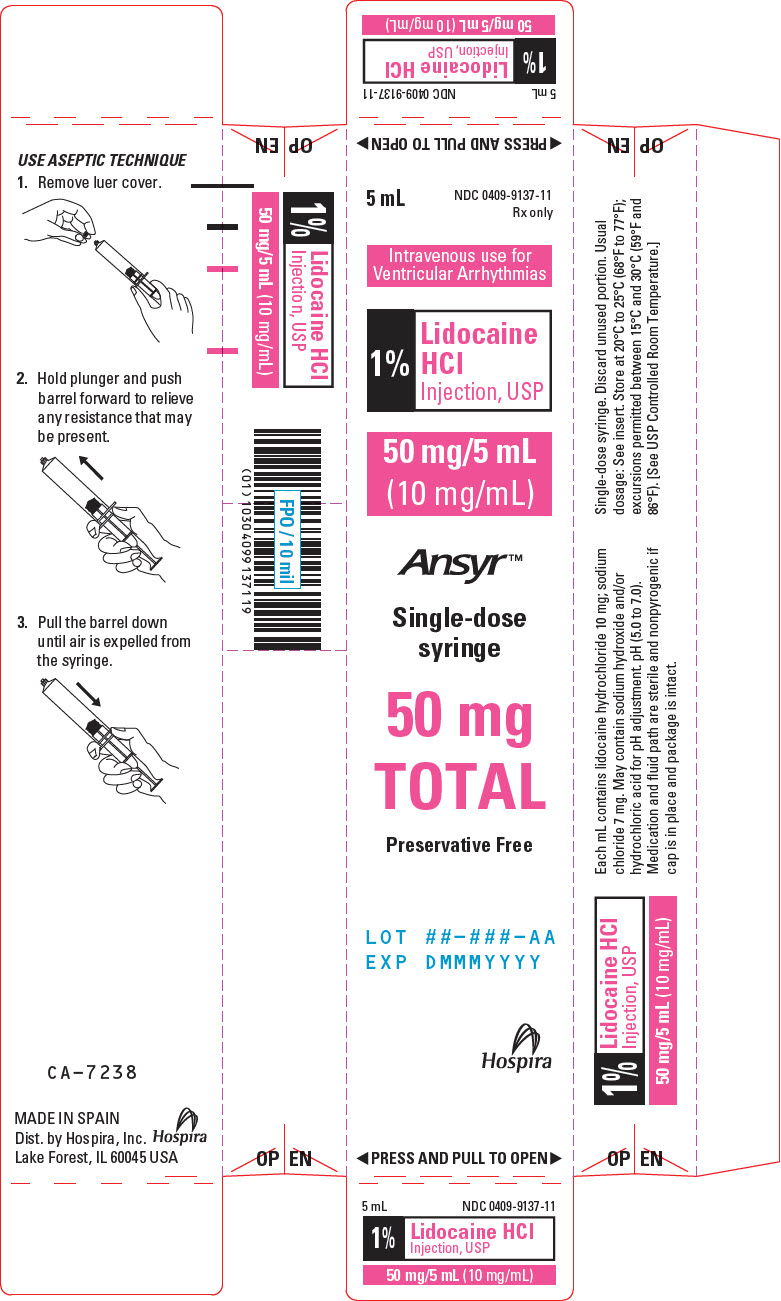
PRINCIPAL DISPLAY PANEL - 10 mg/mL Syringe Carton5 mL - NDC 0409-9137-11 - Rx only - Intravenous use for - Ventricular Arrhythmias - 1% Lidocaine - HCl - Injection, USP - 50 mg/5 mL - (10 mg/mL) Ansyr™ Single-dose - syringe - 50 mg - TOTAL - Preservative Free - LOT ...
-
INGREDIENTS AND APPEARANCEProduct Information