Label: NICOTROL- nicotine spray, metered
- NDC Code(s): 0009-5401-01
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION10 mg/mL
-
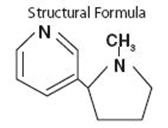
DESCRIPTIONNICOTROL® NS (nicotine nasal spray) is an aqueous solution of nicotine intended for administration as a metered spray to the nasal mucosa. Nicotine is a tertiary amine composed of pyridine and a ...
-
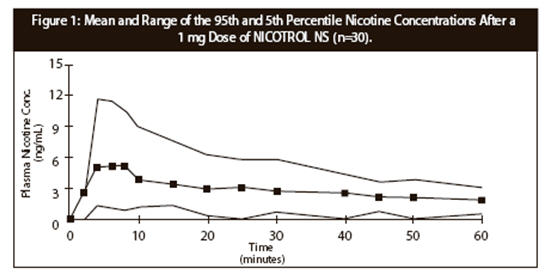
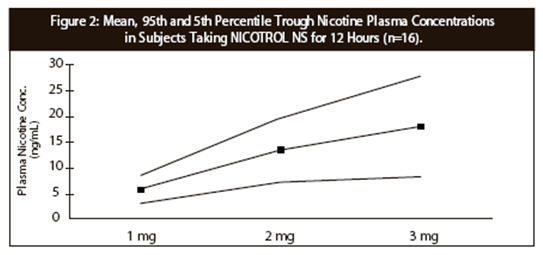
CLINICAL PHARMACOLOGYPharmacologic Action - Nicotine, the chief alkaloid in tobacco products, binds stereo-selectively to nicotinic-cholinergic receptors at the autonomic ganglia, in the adrenal medulla, at ...
-
CLINICAL TRIALSThe efficacy of NICOTROL NS therapy as an aid to smoking cessation was demonstrated in three single-center, placebo-controlled, double-blind trials with a total of 730 patients. One of the trials ...
-
INDICATIONS AND USAGENICOTROL NS is indicated as an aid to smoking cessation for the relief of nicotine withdrawal symptoms. NICOTROL NS therapy should be used as a part of a comprehensive behavioral smoking cessation ...
-
CONTRAINDICATIONSUse of NICOTROL NS therapy is contraindicated in patients with known hypersensitivity or allergy to nicotine or to any component of the product.
-
WARNINGSNicotine from any source can be toxic and addictive. Smoking causes lung disease, cancer, and heart disease and may adversely affect pregnant women or the fetus. For any smoker, with or without ...
-
PRECAUTIONSGeneral - The patient should be urged to stop smoking completely when initiating NICOTROL NS therapy (See DOSAGE AND ADMINISTRATION). Patients should be informed that if they continue to smoke ...
-
ADVERSE REACTIONSAssessment of adverse events in the 730 patients who participated in controlled clinical trials is complicated by the occurrence of signs and symptoms of nicotine withdrawal in some patients and ...
-
DRUG ABUSE AND DEPENDENCENICOTROL NS has a dependence potential intermediate between other nicotine-based therapies and cigarettes. This is the result of differences between cigarettes, NICOTROL NS, nicotine gum and ...
-
OVERDOSAGEThe oral LD50 for nicotine is >5 mg/kg in dogs and >24 mg/kg in rodents. Death is due to respiratory paralysis. The oral minimum acute lethal dose for nicotine in adult humans is reported to be 40 ...
-
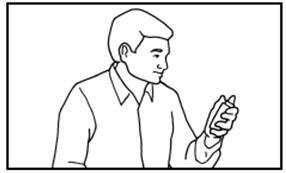
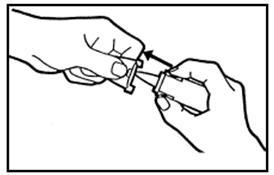
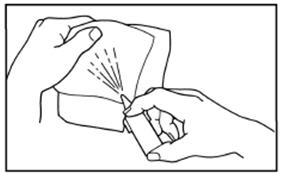
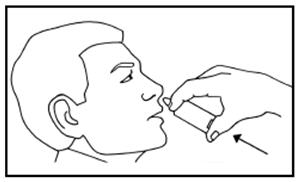
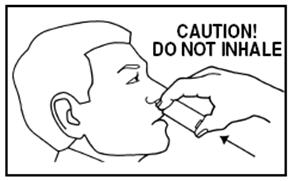
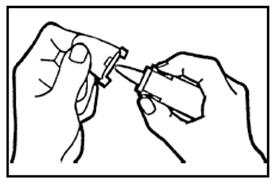
DOSAGE AND ADMINISTRATIONIt is important that patients understand the instructions for use of NICOTROL NS, and have their questions answered. They should clearly understand the directions for using NICOTROL NS and safely ...
-
SAFETY AND HANDLINGAs with all medicines, especially ones in liquid form, care should be taken in handling NICOTROL NS during periods of opening and closing the container (See WARNINGS and Safety Note Concerning ...
-
SPL UNCLASSIFIED SECTIONRx only - LAB-0677-5.0 - Revised June 2024
-
Nicotrol®NS(nicotine nasal spray)An Aid To Help You Stop Smoking - PATIENT INFORMATION - Read and follow carefully. If you have questions or want more information, ask your doctor or pharmacist. IMPORTANT INFORMATION—Read ...
-
SPL UNCLASSIFIED SECTIONLAB-0344-7.0 - Revised June 2024
-
PRINCIPAL DISPLAY PANEL - 10 mg/mL Bottle LabelNDC 0009-5401-01 - Nicotrol® NS - (nicotine - nasal spray) 10 mg/mL - FOR NASAL USE ONLY - KEEP OUT OF REACH OF CHILDREN - 10 mL - Rx only
-
PRINCIPAL DISPLAY PANEL - 10 mg/mL Bottle BoxNDC 0009-5401-01 - Nicotrol® NS - (nicotine - nasal spray - 10 mg/mL - Pharmacist: Place prescription label here. This package contains prescribing information which includes patient instructions.
-
INGREDIENTS AND APPEARANCEProduct Information