Label: KETOCONAZOLE aerosol, foam
- NDC Code(s): 72162-1417-1, 72162-1417-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 45802-532
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KETOCONAZOLE FOAM safely and effectively. See full prescribing information for KETOCONAZOLE FOAM. KETOCONAZOLE foam, 2% For ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEKetoconazole foam, 2% is indicated for the topical treatment of seborrheic dermatitis in immunocompetent patients 12 years of age and older. Limitations of Use - Safety and efficacy of ...
-
2 DOSAGE AND ADMINISTRATIONKetoconazole foam, 2% should be applied to the affected area(s) twice daily for four weeks. Hold the container upright, and dispense ketoconazole foam, 2% into the cap of the can or other cool ...
-
3 DOSAGE FORMS AND STRENGTHSKetoconazole foam, 2% contains 20 mg of ketoconazole, USP per gram, supplied in 50 g and 100 g containers.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Contact Sensitization - Ketoconazole foam, 2% may result in contact sensitization, including photoallergenicity [see Adverse Reactions (6.2)]. 5.2 Flammable Contents - The contents of ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on ketoconazole foam, 2% use in pregnant women to identify a drug-associated risk of major birth defects, miscarriage or adverse ...
-
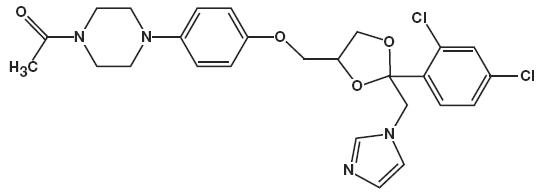
11 DESCRIPTIONKetoconazole foam, 2% contains 2% ketoconazole USP, an antifungal agent, in a thermolabile hydroethanolic foam for topical application. The chemical name for ketoconazole is piperazine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of ketoconazole in the treatment of seborrheic dermatitis is not known. 12.2 Pharmacodynamics - The pharmacodynamics of ketoconazole foam, 2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic or photo-carcinogenic potential of ketoconazole foam ...
-
14 CLINICAL STUDIESThe safety and efficacy of ketoconazole foam, 2% were evaluated in a randomized, double-blind, vehicle-controlled trial in subjects 12 years and older with mild to severe seborrheic dermatitis. In ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGKetoconazole Foam, 2% contains 20 mg of ketoconazole, USP per gram. The thermolabile hydroethanolic foam is available as follows: NDC: 72162-1417-1: 100 g aluminum can - NDC: 72162-1417-2: 50 g ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Package Insert). Instruct patients on a proper use of ketoconazole foam, 2%. • Avoid fire, flame and/or smoking during and immediately following ...
-
SPL UNCLASSIFIED SECTIONManufactured by Padagis® Yeruham, Israel - www.padagis.com - Rev 02-24 - 5K200 RC PH6 - Product of India
-
PATIENT INFORMATIONKetoconazole Foam, 2% Important Information: Ketoconazole foam, 2% is for use on the skin only. Do not use ketoconazole foam, 2% in your eyes, mouth or vagina. What is ketoconazole foam ...
-
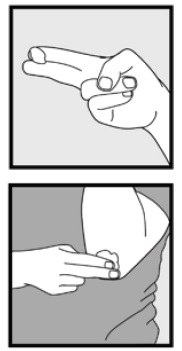
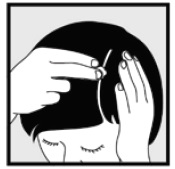
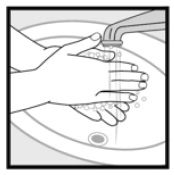
Instructions for Use Ketoconazole Foam, 2% Important Information: Ketoconazole foam, 2% is for use on the skin only. Do not use ketoconazole foam, 2% in your eyes, mouth or vagina. Step 1: Remove the clear cap ...
-
PRINCIPAL DISPLAY PANELKetoconazole 2% Foam
-
INGREDIENTS AND APPEARANCEProduct Information