Label: LAMIVUDINE tablet, film coated
- NDC Code(s): 64380-710-03, 64380-711-04
- Packager: Strides Pharma Science Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LAMIVUDINE TABLETS, safely and effectively. See full prescribing information for LAMIVUDINE TABLETS. LAMIVUDINE tablets, for oral ...These highlights do not include all the information needed to use LAMIVUDINE TABLETS, safely and effectively. See full prescribing information for LAMIVUDINE TABLETS.
LAMIVUDINE tablets, for oral use
Initial U.S. Approval: 1995WARNING: EXACERBATIONS OF HEPATITIS B, and DIFFERENT FORMULATIONS OF LAMIVUDINE
See full prescribing information for complete boxed warning
- Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and human immunodeficiency virus (HIV-1) and have discontinued Lamivudine Monitor hepatic function closely in these patients and, if appropriate, initiate anti-hepatitis B treatment. (5.1)
- Patients with HIV-1 infection should receive only dosage form of Lamivudine appropriate for treatment of HIV-1. (5.1)
RECENT MAJOR CHANGES
Warnings and Precautions, Use with Interferon- and Ribavirin-Based Regimens (previous 5.3) Removed 05/2019
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Adults: 300 mg daily, administered as either 150 mg twice daily or 300 mg once daily. (2.1)
- Pediatric Patients Aged 3 Months and Older: Administered either once or twice daily. Dose should be calculated on body weight (kg) and should not exceed 300 mg daily. (2.2)
- Patients with Renal Impairment: Doses of Lamivudine must be adjusted in accordance with renal function. (2.3)
DOSAGE FORMS AND STRENGTHS
- Tablets: 150 mg scored (3)
- Tablets: 300 mg (3)
CONTRAINDICATIONS
Lamivudine tablets are contraindicated in patients with previous hypersensitivity reaction to lamivudine. (4) (4)
WARNINGS AND PRECAUTIONS
- Co-infected HIV-1/HBV Patients: Emergence of lamivudine-resistant HBV variants associated with lamivudine-containing antiretroviral regimens has been reported. (5.1)
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues. (5.2)
- Pancreatitis: Use with caution in pediatric patients with a history of pancreatitis or other significant risk factors for pancreatitis. Discontinue treatment as clinically appropriate. (5.3)
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. (5.4)
- Lower virologic suppression rates and increased risk of viral resistance were observed in pediatric subjects who received lamivudine oral solution concomitantly with other antiretroviral oral solutions compared with those who received tablets. An all-tablet regimen should be used when possible. (5.5)
ADVERSE REACTIONS
- The most common reported adverse reactions (incidence greater than equal to 15%) in adults were headache, nausea, malaise and fatigue, nasal signs and symptoms, diarrhea, and cough. (6.1)
- The most common reported adverse reactions (incidence greater than equal to 15%) in pediatric patients subjects were fever and cough. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Strides Pharma Inc. at 1-877-244-9825 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch (6)
DRUG INTERACTIONS
Sorbitol: Coadministration of lamivudine and sorbitol may decrease lamivudine concentrations; when possible, avoid chronic coadministration. (7.2) (7)
USE IN SPECIFIC POPULATIONS
- Lactation: Women infected with HIV should be instructed not to breastfeed due to potential for HIV transmission. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EXACERBATIONS OF HEPATITIS B, AND DIFFERENT FORMULATIONS OF LAMIVUDINE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients
2.2 Recommended Dosage for Pediatric Patients
2.3 Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Patients with Hepatitis B Virus Co-infection
5.2 Lactic Acidosis and Severe Hepatomegaly with Steatosis
5.3 Pancreatitis
5.4 Immune Reconstitution Syndrome
5.5 Lower Virologic Suppression Rates and Increased Risk of Viral Resistance with Oral Solution
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Inhibiting Organic Cation Transporters
7.2 Sorbitol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Impaired Renal Function
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Subjects
14.2 Pediatric Subjects
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: EXACERBATIONS OF HEPATITIS B, AND DIFFERENT FORMULATIONS OF LAMIVUDINE
Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and human immunodeficiency virus (HIV-1) and have discontinued lamivudine. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue lamivudine and are co-infected with HIV-1 and HBV. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
Important Differences among Lamivudine-Containing Products
Lamivudine tablets (used to treat HIV-1 infection) contain a higher dose of the active ingredient (lamivudine) than EPIVIR-HBV tablets and oral solution (used to treat chronic HBV infection). Patients with HIV-1 infection should receive only dosage forms appropriate for treatment of HIV-1 [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGELamivudine is a nucleoside analogue indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. Limitations of ...
Lamivudine is a nucleoside analogue indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection.
Limitations of Use:
- The dosage of this product is for HIV-1 and not for HBV.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Adult Patients - The recommended dosage of Lamivudine in HIV-1-infected adults is 300 mg daily, administered as either 150 mg taken orally twice daily or 300 mg taken ...
2.1 Recommended Dosage for Adult Patients
The recommended dosage of Lamivudine in HIV-1-infected adults is 300 mg daily, administered as either 150 mg taken orally twice daily or 300 mg taken orally once daily with or without food. If lamivudine is administered to a patient infected with HIV-1 and HBV, the dosage indicated for HIV-1 therapy should be used as part of an appropriate combination regimen [see Warnings and Precautions (5.1)].
2.2 Recommended Dosage for Pediatric Patients
Lamivudine scored tablet is the preferred formulation for HIV-1-infected pediatric patients who weigh at least 14 kg and for whom a solid dosage form is appropriate. Before prescribing Lamivudine scored tablets, pediatric patients should be assessed for the ability to swallow tablets. For patients unable to safely and reliably swallow Lamivudine tablets, the oral solution formulation may be prescribed [see Warnings and Precautions (5.5)]. The recommended oral dosage of lamivudine tablets for HIV-1-infected pediatric patients is presented in Table 1.
Table 1. Dosing Recommendations for Lamivudine Scored (150-mg) Tablets in Pediatric Patients a Data regarding the efficacy of once-daily dosing is limited to subjects who transitioned from twice-daily dosing to once-daily dosing after 36 weeks of treatment [see Clinical Studies (14.2)].
b Patients may alternatively take one 300-mg tablet, which is not scored.
Weight (kg)
Once-Daily Dosing Regimena
Twice-daily Dosing Regimen Using Scored 150-mg Tablet
AM Dose
PM Dose
Total Daily Dose
14 to <20
1 tablet (150 mg)
½ tablet (75 mg)
½ tablet (75 mg)
150 mg
≥20to <25
1½ tablets (225 mg)
½ tablet (75 mg)
1 tablet (150 mg)
225 mg
≥25
2 tablets (300 mg)b
1 tablet (150 mg)
1 tablet (150 mg)
300 mg
Close2.3 Patients with Renal Impairment
Dosing of Lamivudine is adjusted in accordance with renal function. Dosage adjustments are listed in Table 2 [see Clinical Pharmacology (12.3)].
Table 2. Adjustment of Dosage of Lamivudine tablets in Adults and Adolescents (Greater than or Equal to 25 kg) in Accordance with Creatinine Clearance Creatinine Clearance
(mL/min)
Recommended Dosage of Lamivudine
≥50
150 mg twice daily or 300 mg once daily
30-49
150 mg once daily
15-29
150 mg first dose, then 100 mg once daily
5-14
150 mg first dose, then 50 mg once daily
<5
50 mg first dose, then 25 mg once daily
No additional dosing of Lamivudine tablets is required after routine (4-hour) hemodialysis or peritoneal dialysis.
Although there are insufficient data to recommend a specific dose adjustment of lamivudine tablets in pediatric patients with renal impairment, a reduction in the dose and/or an increase in the dosing interval should be considered.
-
3 DOSAGE FORMS AND STRENGTHSLamivudine Tablets, USP - Scored - Lamivudine scored tablets contains 150 mg of lamivudine. The tablets are white, circular, biconvex, film coated tablets 'TMT' engraved on one side and ...
- Lamivudine Tablets, USP - Scored
Lamivudine scored tablets contains 150 mg of lamivudine. The tablets are white, circular, biconvex, film coated tablets 'TMT' engraved on one side and breakline on other side.
- Lamivudine Tablets, USP
Lamivudine tablets contains 300 mg of lamivudine. The tablets are are white, circular, biconvex, film coated tablets "L300" engraved on one side and plain on other side.
Close -
4 CONTRAINDICATIONSLamivudine Tablets are contraindicated in patients with a previous hypersensitivity reaction to lamivudine.
Lamivudine Tablets are contraindicated in patients with a previous hypersensitivity reaction to lamivudine.
Close -
5 WARNINGS AND PRECAUTIONS5.1 Patients with Hepatitis B Virus Co-infection - Posttreatment Exacerbations of Hepatitis - Clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of ...
5.1 Patients with Hepatitis B Virus Co-infection
Posttreatment Exacerbations of Hepatitis
Clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of lamivudine. These exacerbations have been detected primarily by serum ALT elevations in addition to re-emergence of HBV DNA. Although most events appear to have been self-limited, fatalities have been reported in some cases. Similar events have been reported from postmarketing experience after changes from lamivudine-containing HIV-1 treatment regimens to non-lamivudine-containing regimens in patients infected with both HIV-1 and HBV. The causal relationship to discontinuation of lamivudine treatment is unknown. Patients should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment.
Important Differences among Lamivudine-Containing Products
Lamivudine tablets contain a higher dose of the same active ingredient (lamivudine) than EPIVIR-HBV tablets and EPIVIR-HBV oral solution. EPIVIR-HBV was developed for patients with chronic hepatitis B. The formulation and dosage of lamivudine in EPIVIR-HBV are not appropriate for patients co-infected with HIV-1 and HBV. Safety and efficacy of lamivudine have not been established for treatment of chronic hepatitis B in patients co-infected with HIV-1 and HBV. If treatment with EPIVIR-HBV is prescribed for chronic hepatitis B for a patient with unrecognized or untreated HIV-1 infection, rapid emergence of HIV-1 resistance is likely to result because of the subtherapeutic dose and the inappropriateness of monotherapy HIV-1 treatment. If a decision is made to administer lamivudine to patients co-infected with HIV-1 and HBV, lamivudine tablets, lamivudine oral solution, or another product containing the higher dose of lamivudine should be used as part of an appropriate combination regimen.
Emergence of Lamivudine-Resistant HBV
Safety and efficacy of lamivudine have not been established for treatment of chronic hepatitis B in subjects dually infected with HIV-1 and HBV (see full prescribing information for EPIVIR-HBV). Emergence of hepatitis B virus variants associated with resistance to lamivudine has also been reported in HIV-1-infected subjects who have received lamivudine-containing antiretroviral regimens in the presence of concurrent infection with hepatitis B virus.
5.2 Lactic Acidosis and Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, including lamivudine tablets. A majority of these cases have been in women. Female sex and obesity may be risk factors for the development of lactic acidosis and severe hepatomegaly with steatosis in patients treated with antiretroviral nucleoside analogues. Treatment with lamivudine tablets should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity, which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations.
5.3 Pancreatitis
In pediatric patients with a history of prior antiretroviral nucleoside exposure, a history of pancreatitis, or other significant risk factors for the development of pancreatitis, lamivudine should be used with caution. Treatment with lamivudine should be stopped immediately if clinical signs, symptoms, or laboratory abnormalities suggestive of pancreatitis occur [see Adverse Reactions (6.1)].
5.4 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including lamivudine. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus,Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barre syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Close5.5 Lower Virologic Suppression Rates and Increased Risk of Viral Resistance with Oral Solution
Pediatric subjects who received Lamivudine oral solution (at weight band-based doses approximating 8 mg per kg per day) concomitantly with other antiretroviral oral solutions at any time in the ARROW trial had lower rates of virologic suppression, lower plasma lamivudine exposure, and developed viral resistance more frequently than those receiving Lamivudine tablets [see Clinical Pharmacology (12.3), Microbiology (12.4), Clinical Studies (14.2)].
Lamivudine scored tablet is the preferred formulation for HIV-1-infected pediatric patients who weigh at least 14 kg and for whom a solid dosage form is appropriate. An all-tablet regimen should be used when possible to avoid a potential interaction with sorbitol [see Clinical Pharmacology (12.3)]. Consider more frequent monitoring of HIV-1 viral load when treating with Lamivudine oral solution.
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: Exacerbations of hepatitis B [see Boxed Warning, Warnings and Precautions (5.1)]. Lactic acidosis and severe ...
The following adverse reactions are discussed in other sections of the labeling:
- Exacerbations of hepatitis B [see Boxed Warning, Warnings and Precautions (5.1)].
- Lactic acidosis and severe hepatomegaly with steatosis[see Warnings and Precautions (5.2)].
- Pancreatitis[see Warnings and Precautions (5.3)].
- Immune reconstitution syndrome [see Warnings and Precautions (5.4)].
6.1 Clinical Trials Experience
Clinical Trials Experience in Adult Subjects.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety profile of lamivudine in adults is primarily based on 3,568 HIV-1-infected subjects in 7 clinical trials
The most common adverse reactions are headache, nausea, malaise, fatigue, nasal signs and symptoms, diarrhea and cough.
Selected clinical adverse reactions in greater than or equal to 5% of subjects during therapy with lamivudine 150 mg twice daily plus RETROVIR 200 mg 3 times daily for up to 24 weeks are listed in Table 3.
Table 3. Selected Clinical Adverse Reactions (Greater than or Equal to 5% Frequency) in Four Controlled Clinical Trials (NUCA3001, NUCA3002, NUCB3001, NUCB3002) - *
- Either zidovudine monotherapy or zidovudine in combination with zalcitabine.
Adverse Reaction
Lamivudine 150 mg Twice Daily
plus RETROVIR (n = 251)
RETROVIR*(n = 230)
Body as a Whole
Headache
35%
27%
Malaise & fatigue
27%
23%
Fever or chills
10%
12%
Digestive
Nausea
33%
29%
Diarrhea
18%
22%
Nausea & vomiting
13%
12%
Anorexia and/or decreased appetite
10%
7%
Abdominal pain
9%
11%
Abdominal cramps
6%
3%
Dyspepsia
5%
5%
Nervous System
Neuropathy
12%
10%
Insomnia & other sleep disorders
11%
7%
Dizziness
10%
4%
Depressive disorders
9%
4%
Respiratory
Nasal signs & symptoms
20%
11%
Cough
18%
13%
Skin
Skin rashes
9%
6%
Musculoskeletal
Musculoskeletal pain
12%
10%
Myalgia
8%
6%
Arthralgia
5%
5%
Pancreatitis: Pancreatitis was observed in 9 out of 2,613 adult subjects (0.3%) who received lamivudine in controlled clinical trials EPV20001, NUCA3001, NUCB3001, NUCA3002, NUCB3002, and NUCB3007[see Warnings and Precautions (5.4)].
Lamivudine 300 mg Once Daily: The types and frequencies of clinical adverse reactions reported in subjects receiving lamivudine 300 mg once daily or lamivudine 150 mg twice daily (in 3-drug combination regimens in EPV20001 and EPV40001) for 48 weeks were similar.
Selected laboratory abnormalities observed during therapy are summarized in Table 4.
Table 4. Frequencies of Selected Grade 3-4 Laboratory Abnormalities in Adults in Four 24-Week Surrogate Endpoint Trials (NUCA3001, NUCA3002, NUCB3001, NUCB3002) and a Clinical Endpoint Trial (NUCB3007) ULN = Upper limit of normal.
ND = Not done.
Test
(Threshold Level)
24-Week Surrogate Endpoint Trials*
Clinical Endpoint Trials*
Lamivudine
Plus
RETROVIR
RETROVIR†
Lamivudine
plus Current
Therapy‡
Placebo plus
Current
Therapy‡
Absolute neutrophil count
(<750/mm3)
7.2%
5.4
15%
13%
Hemoglobin (<8.0 g/dL)
2.9%
1.8
2.2%
3.4%
Platelets (<50,000/mm3)
0.4%
1.3
2.8%
3.8%
ALT (>5.0 x ULN)
3.7%
3.6
3.8%
1.9%
AST (>5.0 x ULN)
1.7%
1.8
4.0%
2.1%
Bilirubin (>2.5 x ULN)
0.8%
0.4
ND
ND
Amylase (>2.0 x ULN)
4.2%
1.5
2.2%
1.1%
The frequencies of selected laboratory abnormalities reported in subjects receiving lamivudine 300 mg once daily or lamivudine 150 mg twice daily (in 3-drug combination regimens in EPV20001 and EPV40001) were similar.
Clinical Trials Experience in Pediatric Subjects
Lamivudine oral solution has been studied in 638 pediatric subjects aged 3 months to 18 years in 3 clinical trials.
Selected clinical adverse reactions and physical findings with a greater than or equal to 5% frequency during therapy with lamivudine 4 mg per kg twice daily plus RETROVIR 160 mg per m2 3 times daily in therapy-naive (less than or equal to 56 days of antiretroviral therapy) pediatric subjects are listed in Table 5.
Table 5. Selected Clinical Adverse Reactions and Physical Findings (Greater than or Equal to 5% Frequency) in Pediatric Subjects in Trial ACTG300 - *
- Includes pain, discharge, erythema, or swelling of an ear.
Adverse Reaction
Lamivudine plus RETROVIR (n = 236)
Didanosine (n = 235)
Body as a Whole
Fever
25%
32%
Digestive
Hepatomegaly
11%
11%
Nausea & vomiting
8%
7%
Diarrhea
8%
6%
Stomatitis
6%
12%
Splenomegaly
5%
8%
Respiratory
Cough
15%
18%
Abnormal breath sounds/wheezing
7%
9%
Ear, Nose and Throat
Signs or symptoms of ears*
Nasal discharge or congestion
7%
6%
8%
11%
Other
Skin rashes
12%
14%
Lymphadenopathy
9%
11%
Pancreatitis: Pancreatitis, which has been fatal in some cases, has been observed in antiretroviral nucleoside-experienced pediatric subjects receiving lamivudine alone or in combination with other antiretroviral agents. In an open-label dose-escalation trial (NUCA2002), 14 subjects (14%) developed pancreatitis while receiving monotherapy with lamivudine. Three of these subjects died of complications of pancreatitis. In a second open-label trial (NUCA2005), 12 subjects (18%) developed pancreatitis. In Trial ACTG300, pancreatitis was not observed in 236 subjects randomized to lamivudine plus RETROVIR. Pancreatitis was observed in 1 subject in this trial who received open-label lamivudine in combination with RETROVIR and ritonavir following discontinuation of didanosine monotherapy [see Warnings and Precautions (5.3)].
Paresthesias and Peripheral Neuropathies: Paresthesias and peripheral neuropathies were reported in 15 subjects (15%) in Trial NUCA2002, 6 subjects (9%) in Trial NUCA2005, and 2 subjects (less than 1%) in Trial ACTG300.
Selected laboratory abnormalities experienced by therapy-naive (less than or equal to 56 days of antiretroviral therapy) pediatric subjects are listed in Table 6.
Table 6. Frequencies of Selected Grade 3-4 Laboratory Abnormalities in Pediatric Subjects in Trial ACTG300 ULN = Upper limit of normal.
Test (Threshold Level)
Lamivudine
plus
RETROVIR
Didanosine
Absolute neutrophil count (<400/mm3)
8%
3%
Hemoglobin (<7.0 g/dL)
4%
2%
Platelets (<50,000/mm3)
1%
3%
ALT (>10 x ULN)
1%
3%
AST (>10 x ULN)
2%
4%
Lipase (>2.5 x ULN)
3%
3%
Total Amylase (>2.5 x ULN)
3%
3%
Pediatric Subjects Once-Daily versus Twice-Daily Dosing (COL105677): The safety of once-daily compared with twice-daily dosing of lamivudine was assessed in the ARROW trial. Primary safety assessment in the ARROW trial was based on Grade 3 and Grade 4 adverse events. The frequency of Grade 3 and 4 adverse events was similar among subjects randomized to once-daily dosing compared with subjects randomized to twice-daily dosing. One event of Grade 4 hepatitis in the once-daily cohort was considered as uncertain causality by the investigator and all other Grade 3 or 4 adverse events were considered not related by the investigator.
Neonates: Limited short-term safety information is available from 2 small, uncontrolled trials in South Africa in neonates receiving lamivudine with or without zidovudine for the first week of life following maternal treatment starting at Week 38 or 36 of gestation [see Clinical Pharmacology (12.3)]. Selected adverse reactions reported in these neonates included increased liver function tests, anemia, diarrhea, electrolyte disturbances, hypoglycemia, jaundice and hepatomegaly, rash, respiratory infections, and sepsis; 3 neonates died (1 from gastroenteritis with acidosis and convulsions, 1 from traumatic injury, and 1 from unknown causes). Two other nonfatal gastroenteritis or diarrhea cases were reported, including 1 with convulsions; 1 infant had transient renal insufficiency associated with dehydration. The absence of control groups limits assessments of causality, but it should be assumed that perinatally exposed infants may be at risk for adverse reactions comparable to those reported in pediatric and adult HIV-1-infected patients treated with lamivudine-containing combination regimens. Long-term effects of in utero and infant lamivudine exposure are not known.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of lamivudine. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to lamivudine.
Body as a Whole
Redistribution/accumulation of body fat.
Endocrine and Metabolic
Hyperglycemia.
General
Weakness.
Hemic and Lymphatic
Anemia (including pure red cell aplasia and severe anemias progressing on therapy).
Hepatic and Pancreatic
Lactic acidosis and hepatic steatosis [see Warnings and Precautions (5.2)], posttreatment exacerbations of hepatitis B [see Warnings and Precautions (5.1)].
Hypersensitivity
Anaphylaxis, urticaria.
Musculoskeletal
Muscle weakness, CPK elevation, rhabdomyolysis.
Skin
Alopecia, pruritus.
-
7 DRUG INTERACTIONS7.1 Drugs Inhibiting Organic Cation Transporters - Lamivudine is predominantly eliminated in the urine by active organic cationic secretion. The possibility of interactions with other drugs ...
7.1 Drugs Inhibiting Organic Cation Transporters
Lamivudine is predominantly eliminated in the urine by active organic cationic secretion. The possibility of interactions with other drugs administered concurrently should be considered, particularly when their main route of elimination is active renal secretion via the organic cationic transport system (e.g., trimethoprim)[see Pharmacokinetics (12.3)]. No data are available regarding interactions with other drugs that have renal clearance mechanisms similar to that of lamivudine.
Close7.2 Sorbitol
Coadministration of single doses of lamivudine and sorbitol resulted in a sorbitol dose-dependent reduction in lamivudine exposures. When possible, avoid use of sorbitol-containing medicines with lamivudine [see Warnings and Precautions (5.5), Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Lamivudine during pregnancy. Healthcare providers are ...
8.1 Pregnancy
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Lamivudine during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Available data from the APR show no difference in the overall risk of birth defects for lamivudine compared with the background rate for birth defects of 2.7% in the Metropolitan Atlanta Congenital Defects Program (MACDP) reference population (see Data). The APR uses the MACDP as the U.S. reference population for birth defects in the general population. The MACDP evaluates women and infants from a limited geographic area and does not include outcomes for births that occurred at less than 20 weeks' gestation. The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15% to 20%. The background risk for major birth defects and miscarriage for the indicated population is unknown.
In animal reproduction studies, oral administration of lamivudine to pregnant rabbits during organogenesis resulted in embryolethality at systemic exposure (AUC) similar to the recommended clinical dose; however, no adverse development effects were observed with oral administration of lamivudine to pregnant rats during organogenesis at plasma concentrations (Cmax) 35 times the recommended clinical dose (see Data).
Data
Human Data: Based on prospective reports from the APR of over 11,000 exposures to lamivudine during pregnancy resulting in live births (including over 4,500 exposed in the first trimester), there was no difference between the overall risk of birth defects for lamivudine compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of defects in live births was 3.1% (95% CI: 2.6% to 3.6%) following first trimester exposure to lamivudine-containing regimens and 2.8% (95% CI: 2.5% to 3.3%) following second/third trimester exposure to lamivudine-containing regimens.
Lamivudine pharmacokinetics were studied in pregnant women during 2 clinical trials conducted in South Africa. The trials assessed pharmacokinetics in 16 women at 36 weeks gestation using 150 mg lamivudine twice daily with zidovudine, 10 women at 38 weeks gestation using 150 mg lamivudine twice daily with zidovudine, and 10 women at 38 weeks gestation using lamivudine 300 mg twice daily without other antiretrovirals. These trials were not designed or powered to provide efficacy information. Lamivudine concentrations were generally similar in maternal, neonatal, and umbilical cord serum samples. In a subset of subjects, amniotic fluid specimens were collected following natural rupture of membranes and confirmed that lamivudine crosses the placenta in humans. Based on limited data at delivery, median (range) amniotic fluid concentrations of lamivudine were 3.9 (1.2 to 12.8)–fold greater compared with paired maternal serum concentration (n = 8).
Animal Data: Lamivudine was administered orally to pregnant rats (at 90, 600, and 4,000 mg per kg per day) and rabbits (at 90, 300, and 1,000 mg per kg per day and at 15, 40, and 90 mg per kg per day) during organogenesis (on gestation Days 7 through 16 [rat] and 8 through 20 [rabbit]). No evidence of fetal malformations due to lamivudine was observed in rats and rabbits at doses producing plasma concentrations (Cmax) approximately 35 times higher than human exposure at the recommended daily dose. Evidence of early embryolethality was seen in the rabbit at system exposures (AUC) similar to those observed in humans, but there was no indication of this effect in the rat at plasma concentrations (Cmax) 35 times higher than human exposure at the recommended daily dose. Studies in pregnant rats showed that lamivudine is transferred to the fetus through the placenta. In the fertility/pre-and postnatal development study in rats, lamivudine was administered orally at doses of 180, 900, and 4,000 mg per kg per day (from prior to mating through postnatal Day 20). In the study, development of the offspring, including fertility and reproductive performance, was not affected by maternal administration of lamivudine.
8.2 Lactation
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection. Lamivudine is present in human milk. There is no information on the effects of lamivudine on the breastfed infant or the effects of the drugs on milk production. Because of the potential for (1) HIV-1 transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants), and (3) adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving lamivudine tablets.
8.4 Pediatric Use
The safety and effectiveness of lamivudine in combination with other antiretroviral agents have been established in pediatric patients aged 3 months and older. Lamivudine scored tablet is the preferred formulation for HIV-1-infected pediatric patients who weigh at least 14 kg and for whom a solid dosage form is appropriate because pediatric subjects who received Lamivudine oral solution had lower rates of virologic suppression, lower plasma lamivudine exposure, and developed viral resistance more frequently than those receiving lamivudine tablets in the ARROW trial [see Dosage and Administration (2.2), Warnings and Precautions (5.5), Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.2)].
8.5 Geriatric Use
Clinical trials of lamivudine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration of lamivudine in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
Close8.6 Patients with Impaired Renal Function
Reduction of the dosage of lamivudine is recommended for patients with impaired renal function [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEThere is no known specific treatment for overdose with lamivudine. If overdose occurs, the patient should be monitored, and standard supportive treatment applied as required. Because a negligible ...
There is no known specific treatment for overdose with lamivudine. If overdose occurs, the patient should be monitored, and standard supportive treatment applied as required. Because a negligible amount of lamivudine was removed via (4-hour) hemodialysis, continuous ambulatory peritoneal dialysis, and automated peritoneal dialysis, it is not known if continuous hemodialysis would provide clinical benefit in a lamivudine overdose event.
Close -
11 DESCRIPTIONLamivudine (also known as 3TC), a synthetic nucleoside analogue with activity against HIV-1 and HBV. The chemical name of lamivudine is ...
Lamivudine (also known as 3TC), a synthetic nucleoside analogue with activity against HIV-1 and HBV. The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-) enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)2′,3′-dideoxy, 3′-thiacytidine. It has a molecular formula of C8H11N3O3S and a molecular weight of 229.3 g per mol. It has the following structural formula:
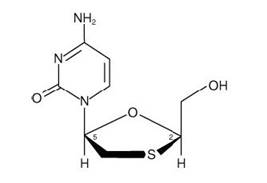
Lamivudine is a white to off-white crystalline solid with a solubility of approximately 70 mg per mL in water at 20°C.
Lamivudine Tablets, USP are for oral administration. Each scored 150-mg film-coated tablet contains 150 mg of lamivudine and the inactive ingredients microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, povidone, magnesium stearate, and opadry white which is composed of Hydroxy Propyl methylcellulose 2910/ Hypromellose 5cP, Titanium dioxide, Polyethylene glycol 400 (Macrogol).
Each 300-mg film-coated tablet contains 300 mg of lamivudine and the inactive ingredients microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, povidone, magnesium stearate, and opadry white which is composed of Hydroxy Propyl methylcellulose 2910/ Hypromellose 5cP, Titanium dioxide, Polyethylene glycol 400 (Macrogol).
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lamivudine is an antiretroviral agent [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Pharmacokinetics in Adults - The pharmacokinetic properties of lamivudine ...
12.3 Pharmacokinetics
The pharmacokinetic properties of lamivudine have been studied in asymptomatic, HIV-1-infected adult subjects after administration of single intravenous (IV) doses ranging from 0.25 to 8 mg per kg, as well as single and multiple (twice-daily regimen) oral doses ranging from 0.25 to 10 mg per kg.
The pharmacokinetic properties of lamivudine have also been studied as single and multiple oral doses ranging from 5 mg to 600 mg per day administered to HBV-infected subjects.
The steady-state pharmacokinetic properties of the lamivudine 300-mg tablet once daily for 7 days compared with the lamivudine 150-mg tablet twice daily for 7 days were assessed in a crossover trial in 60 healthy subjects. Lamivudine 300 mg once daily resulted in lamivudine exposures that were similar to lamivudine 150 mg twice daily with respect to plasma AUC24,ss; however, Cmax,ss was 66% higher and the trough value was 53% lower compared with the 150-mg twice-daily regimen. Intracellular lamivudine triphosphate exposures in peripheral blood mononuclear cells were also similar with respect to AUC24,ss and Cmax24,ss; however, trough values were lower compared with the 150-mg twice-daily regimen. Inter-subject variability was greater for intracellular lamivudine triphosphate concentrations versus lamivudine plasma trough concentrations.
The pharmacokinetics of lamivudine was evaluated in 12 adult HIV-1-infected subjects dosed with lamivudine 150 mg twice daily in combination with other antiretroviral agents. The geometric mean (95% CI) for AUC(0-12) was 5.53 (4.58, 6.67) mcg.h per mL and for Cmax was 1.40 (1.17, 1.69) mcg per mL.
Absorption and Bioavailability: Absolute bioavailability in 12 adult subjects was 86% ± 16% (mean ± SD) for the 150-mg tablet and 87% ± 13% for the oral solution. After oral administration of 2 mg per kg twice a day to 9 adults with HIV-1, the peak serum lamivudine concentration (Cmax) was 1.5 ± 0.5 mcg per mL (mean ± SD). The area under the plasma concentration versus time curve (AUC) and Cmax increased in proportion to oral dose over the range from 0.25 to 10 mg per kg.
The accumulation ratio of lamivudine in HIV-1-positive asymptomatic adults with normal renal function was 1.50 following 15 days of oral administration of 2 mg per kg twice daily.
Effects of Food on Oral Absorption: Lamivudine tablets may be administered with or without food. An investigational 25-mg dosage form of lamivudine was administered orally to 12 asymptomatic, HIV-1-infected subjects on 2 occasions, once in the fasted state and once with food (1,099 kcal; 75 grams fat, 34 grams protein, 72 grams carbohydrate). Absorption of lamivudine was slower in the fed state (Tmax: 3.2 ±1.3 hours) compared with the fasted state (Tmax: 0.9 ± 0.3 hours); Cmax in the fed state was 40% ± 23% (mean ± SD) lower than in the fasted state. There was no significant difference in systemic exposure (AUC∞) in the fed and fasted states.
Distribution: The apparent volume of distribution after IV administration of lamivudine to 20 subjects was 1.3 ± 0.4 L per kg, suggesting that lamivudine distributes into extravascular spaces. Volume of distribution was independent of dose and did not correlate with body weight.
Binding of lamivudine to human plasma proteins is less than 36%. In vitro studies showed that over the concentration range of 0.1 to 100 mcg per mL, the amount of lamivudine associated with erythrocytes ranged from 53% to 57% and was independent of concentration.
Metabolism: Metabolism of lamivudine is a minor route of elimination. In humans, the only known metabolite of lamivudine is the trans-sulfoxide metabolite (approximately 5% of an oral dose after 12 hours). Serum concentrations of this metabolite have not been determined. Lamivudine is not significantly metabolized by cytochrome P450 enzymes.
Elimination: The majority of lamivudine is eliminated unchanged in urine by active organic cationic secretion. In 9 healthy subjects given a single 300-mg oral dose of lamivudine, renal clearance was 199.7 ± 56.9 mL per min (mean SD). In 20 HIV-1-infected subjects given a single IV dose, renal clearance was 280.4 ± 75.2 mL per min (mean SD), representing 71% ± 16% (mean ± SD) of total clearance of lamivudine.
In most single-dose trials in HIV-1-infected subjects, HBV-infected subjects, or healthy subjects with serum sampling for 24 hours after dosing, the observed mean elimination half-life (t½) ranged from 5 to 7 hours. In HIV-1-infected subjects, total clearance was 398.5 ± 69.1 mL per min (mean ± SD). Oral clearance and elimination half-life were independent of dose and body weight over an oral dosing range of 0.25 to 10 mg per kg.
Specific Populations
Patients with Renal Impairment: The pharmacokinetic properties of lamivudine have been determined in a small group of HIV-1-infected adults with impaired renal function (Table 7).
Table 7. Pharmacokinetic Parameters (Mean ± SD) after a Single 300-mg Oral Dose of Lamivudine in 3 Groups of Adults with Varying Degrees of Renal Function Parameter
Creatinine Clearance Criterion (Number of Subjects)
>60 mL/min
(n = 6)
10-30 mL/min
(n = 4)
<10 mL/min
(n = 6)
Creatinine clearance (mL/min)
111 ± 14
28 ± 8
6 ± 2
Cmax (mcg/mL)
2.6 ± 0.5
3.6 ± 0.8
5.8 ± 1.2
AUC∞ (mcg•h/mL)
11.0 ± 1.7
48.0 ± 19
157 ± 74
Cl/F (mL/min)
464 ± 76
114 ± 34
36 ± 11
Tmax was not significantly affected by renal function. Based on these observations, it is recommended that the dosage of lamivudine be modified in patients with renal impairment [see Dosage and Administration (2.3)].
Based on a trial in otherwise healthy subjects with impaired renal function, hemodialysis increased lamivudine clearance from a mean of 64 to 88 mL per min; however, the length of time of hemodialysis (4 hours) was insufficient to significantly alter mean lamivudine exposure after a single-dose administration. Continuous ambulatory peritoneal dialysis and automated peritoneal dialysis have negligible effects on lamivudine clearance. Therefore, it is recommended, following correction of dose for creatinine clearance, that no additional dose modification be made after routine hemodialysis or peritoneal dialysis.
The effects of renal impairment on lamivudine pharmacokinetics in pediatric patients are not known.
Patients with Hepatic Impairment: The pharmacokinetic properties of lamivudine have been determined in adults with impaired hepatic function. Pharmacokinetic parameters were not altered by diminishing hepatic function. Safety and efficacy of lamivudine have not been established in the presence of decompensated liver disease.
Pregnant Women: Lamivudine pharmacokinetics were studied in 36 pregnant women during 2 clinical trials conducted in South Africa. Lamivudine pharmacokinetics in pregnant women were similar to those seen in non-pregnant adults and in postpartum women. Lamivudine concentrations were generally similar in maternal, neonatal, and umbilical cord serum samples.
Pediatric Patients: The pharmacokinetics of lamivudine have been studied after either single or repeat doses of lamivudine tablets in 210 pediatric subjects. Pediatric subjects receiving lamivudine oral solution (dosed at approximately 8 mg per kg per day) achieved approximately 25% lower plasma concentrations of lamivudine compared with HIV-1-infected adults. Pediatric subjects receiving lamivudine oral tablets achieved plasma concentrations comparable to or slightly higher than those observed in adults. The absolute bioavailability of both lamivudine tablets and oral solution are lower in children than adults. The relative bioavailability of lamivudine oral solution is approximately 40% lower than tablets containing lamivudine in pediatric subjects despite no difference in adults. Lower lamivudine exposures in pediatric patients receiving lamivudine oral solution is likely due to the interaction between lamivudine and concomitant solutions containing sorbitol (such as ZIAGEN). Modeling of pharmacokinetic data suggests increasing the dosage of lamivudine oral solution to 5 mg per kg taken orally twice daily or 10 mg per kg taken orally once daily (up to a maximum of 300 mg daily) is needed to achieve sufficient concentrations of lamivudine [see Dosage and Administration (2.2)]. There are no clinical data in HIV-1 infected pediatric patients coadministered with sorbitol-containing medicines at this dose.
The pharmacokinetics of lamivudine dosed once daily in HIV-1-infected pediatric subjects aged 3 months through 12 years was evaluated in 3 trials (PENTA-15 [n = 17], PENTA 13 [n = 19], and ARROW PK [n = 35]). All 3 trials were 2-period, crossover, open-label pharmacokinetic trials of twice-versus once-daily dosing of abacavir and lamivudine. These 3 trials demonstrated that once-daily dosing provides similar AUC0-24 to twice-daily dosing of lamivudine at the same total daily dose when comparing the dosing regimens within the same formulation (i.e., either the oral solution or the tablet formulation). The mean Cmax was approximately 80% to 90% higher with lamivudine once-daily dosing compared with twice-daily dosing.
Table 8. Pharmacokinetic Parameters (Geometric Mean [95% CI]) after Repeat Dosing of Lamivudine in 3 Pediatric Trials a n = 16 for PENTA-15 Cmax
b Solution was dosed at 8 mg per kg per day.
c Five subjects in PENTA-13 received lamivudine tablets.
Trial
(Number of Subjects)
ARROW PK
(n = 35)
PENTA-13
(n = 19)
PENTA-15
(n = 17)a
Age Range
3-12 years
2-12 years
3-36 months
Formulation
Tablet
Solutionb and Tabletc
Solutionb
Parameter
Once Daily
Twice Daily
Once Daily
Twice Daily
Once Daily
Twice Daily
Cmax (mcg/mL)
3.17
(2.76, 3.64)
1.80 (1.59, 2.04)
2.09 (1.80, 2.42)
1.11 (0.96, 1.29)
1.87 (1.65, 2.13)
1.05 (0.88, 1.26)
AUC(0-24) (mcg•h/mL)
13.0 (11.4, 14.9)
12.0 (10.7, 13.4)
9.80 (8.64, 11.1)
8.88 (7.67, 10.3)
8.66 (7.46, 10.1)
9.48 (7.89, 11.4)
Distribution of lamivudine into cerebrospinal fluid (CSF) was assessed in 38 pediatric subjects after multiple oral dosing with lamivudine. CSF samples were collected between 2 and 4 hours postdose. At the dose of 8 mg per kg per day, CSF lamivudine concentrations in 8 subjects ranged from 5.6% to 30.9% (mean ± SD of 14.2% ± 7.9%) of the concentration in a simultaneous serum sample, with CSF lamivudine concentrations ranging from 0.04 to 0.3 mcg per mL.
Limited, uncontrolled pharmacokinetic and safety data are available from administration of lamivudine (and zidovudine) to 36 infants aged up to 1 week in 2 trials in South Africa. In these trials, lamivudine clearance was substantially reduced in 1-week-old neonates relative to pediatric subjects (aged over 3 months) studied previously. There is insufficient information to establish the time course of changes in clearance between the immediate neonatal period and the age-ranges over 3 months old [see Adverse Reactions (6.1)].
Geriatric Patients: The pharmacokinetics of lamivudine after administration of lamivudine tablets to subjects over 65 years have not been studied [see Use in Specific Populations (8.5)].
Male and Female Patients: There are no significant or clinically relevant gender differences in lamivudine pharmacokinetics
Racial Groups: There are no significant or clinically relevant racial differences in lamivudine pharmacokinetics.
Drug Interaction Studies
Effect of Lamivudine on the Pharmacokinetics of Other Agents: Based on in vitro study results, lamivudine at therapeutic drug exposures is not expected to affect the pharmacokinetics of drugs that are substrates of the following transporters: organic anion transporter polypeptide 1B1/3 (OATP1B1/3), breast cancer resistance protein (BCRP), P-glycoprotein (P-gp), multidrug and toxin extrusion protein 1 (MATE1), MATE2-K, organic cation transporter 1 (OCT1), OCT2, or OCT3.
Effect of Other Agents on the Pharmacokinetics of Lamivudine: Lamivudine is a substrate of MATE1, MATE2-K, and OCT2 in vitro. Trimethoprim (an inhibitor of these drug transporters) has been shown to increase lamivudine plasma concentrations. This interaction is not considered clinically significant as no dose adjustment of lamivudine is needed.
Lamivudine is a substrate of P-gp and BCRP; however, considering its absolute bioavailability (87%), it is unlikely that these transporters play a significant role in the absorption of lamivudine. Therefore, coadministration of drugs that are inhibitors of these efflux transporters is unlikely to affect the disposition and elimination of lamivudine.
Interferon Alfa: There was no significant pharmacokinetic interaction between lamivudine and interferon alfa in a trial of 19 healthy male subjects.
Ribavirin: In vitro data indicate ribavirin reduces phosphorylation of lamivudine, stavudine, and zidovudine. However, no pharmacokinetic (e.g., plasma concentrations or intracellular triphosphorylated active metabolite concentrations) or pharmacodynamic (e.g., loss of HIV-1/HCV virologic suppression) interaction was observed when ribavirin and lamivudine (n=18), stavudine (n = 10), or zidovudine (n = 6) were coadministered as part of a multi-drug regimen to HIV-1/HCV co-infected subjects.
Sorbitol (Excipient): Lamivudine and sorbitol solutions were coadministered to 16 healthy adult subjects in an open-label, randomized sequence, 4-period, crossover trial. Each subject received a single 300-mg dose of lamivudine oral solution alone or coadministered with a single dose of 3.2 grams, 10.2 grams, or 13.4 grams of sorbitol in solution. Coadministration of lamivudine with sorbitol resulted in dose-dependent decreases of 20%, 39%, and 44% in the AUC(0-24), 14%, 32%, and 36% in the AUC(∞), and 28%, 52%, and 55% in the Cmax of lamivudine, respectively.
Trimethoprim/Sulfamethoxazole: Lamivudine and TMP/SMX were coadministered to 14 HIV-1-positive subjects in a single-center, open-label, randomized, crossover trial. Each subject received treatment with a single 300-mg dose of lamivudine and TMP 160 mg/SMX 800 mg once a day for 5 days with concomitant administration of lamivudine 300 mg with the fifth dose in a crossover design. Coadministration of TMP/SMX with lamivudine resulted in an increase of 43% ± 23% (mean SD) in lamivudine AUC∞, a decrease of 29% ± 13% in lamivudine oral clearance, and a decrease of 30% ± 36% in lamivudine renal clearance. The pharmacokinetic properties of TMP and SMX were not altered by coadministration with lamivudine. There is no information regarding the effect on lamivudine pharmacokinetics of higher doses of TMP/SMX such as those used in treat PCP.
Zidovudine: No clinically significant alterations in lamivudine or zidovudine pharmacokinetics were observed in 12 asymptomatic HIV-l-infected adult subjects given a single dose of zidovudine (200 mg) in combination with multiple doses of lamivudine (300 mg every 12 hours).
Close12.4 Microbiology
Lamivudine is a synthetic nucleoside analogue. Intracellularly, lamivudine is phosphorylated to its active 5′-triphosphate metabolite, lamivudine triphosphate (3TC-TP). The principal mode of action of 3TC-TP is inhibition of HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation of the nucleotide analogue.
Antiviral Activity
The antiviral activity of lamivudine against HIV-1 was assessed in a number of cell lines including monocytes and fresh human peripheral blood lymphocytes (PBMCs) using standard susceptibility assays. EC50 values were in the range of 0.003 to 15 microM (1 microM = 0.23 mcg per mL). The median EC50 values of lamivudine were 60 nM (range: 20 to 70 nM), 35 nM (range: 30 to 40 nM), 30 nM (range: 20 to 90 nM), 20 nM (range: 3 to 40 nM), 30 nM (range: 1 to 60 nM), 30 nM (range: 20 to 70 nM), 30 nM (range: 3 to 70 nM), and 30 nM (range: 20 to 90 nM) against HIV-1 clades A-G and group O viruses (n = 3 except n = 2 for clade B) respectively. The EC50 values against HIV-2 isolates (n = 4) from 0.003 to 0.120 microM in PBMCs. Lamivudine was not antagonistic to all tested anti-HIV agents. Ribavirin (50 microM) used in the treatment of chronic HCV infection decreased the anti-HIV-1 activity of lamivudine by 3.5-fold in MT-4 cells.
Resistance
Lamivudine-resistant variants of HIV-1 have been selected in cell culture. Genotypic analysis showed that the resistance was due to a specific amino acid substitution in the HIV-1 reverse transcriptase at codon 184 changing the methionine to either isoleucine or valine (M184V/I).
HIV-1 strains resistant to both lamivudine and zidovudine have been isolated from subjects. Susceptibility of clinical isolates to lamivudine and zidovudine was monitored in controlled clinical trials. In subjects receiving lamivudine monotherapy or combination therapy with lamivudine plus zidovudine, HIV-1 isolates from most subjects became phenotypically and genotypically resistant to lamivudine within 12 weeks.
Genotypic and Phenotypic Analysis of On-therapy HIV-1 Isolates from Subjects with Virologic Failure
Trial EPV20001: Fifty-three of 554 (10%) subjects enrolled in EPV20001 were identified as virological failures (plasma HIV-1 RNA level greater than or equal to 400 copies per mL) by Week 48. Twenty-eight subjects were randomized to the lamivudine once-daily treatment group and 25 to the lamivudine twice-daily treatment group. The median baseline plasma HIV-1 RNA levels of subjects in the lamivudine once-daily group and lamivudine twice-daily group were 4.9 log10 copies per mL and 4.6 log10 copies per mL, respectively.
Genotypic analysis of on-therapy isolates from 22 subjects identified as virologic failures in the lamivudine once-daily group showed that isolates from 8 of 22 subjects contained a treatment-emergent lamivudine resistance-associated substitution (M184V or M184I), isolates from 0 of 22 subjects contained treatment-emergent amino acid substitutions associated with zidovudine resistance (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E), and isolates from 10 of 22 subjects contained treatment-emergent amino acid substitutions associated with efavirenz resistance (L100I, K101E, K103N, V108I, or Y181C).
Genotypic analysis of on-therapy isolates from subjects (n = 22) in the lamivudine twice-daily treatment group showed that isolates from 5 of 22 subjects contained treatment-emergent lamivudine resistance substitutions, isolates from 1 of 22 subjects contained treatment-emergent zidovudine resistance substitutions, and isolates from 7 of 22 subjects contained treatment-emergent efavirenz resistance substitutions.
Phenotypic analysis of baseline-matched on-therapy HIV-1 isolates from subjects (n = 13) receiving lamivudine once daily showed that isolates from 7 of 13 subjects showed an 85- to 299-fold decrease in susceptibility to lamivudine, isolates from 12 of 13 subjects were susceptible to zidovudine, and isolates from 8 of 13 subjects exhibited a 25- to 295-fold decrease in susceptibility to efavirenz.
Phenotypic analysis of baseline-matched on-therapy HIV-1 isolates from subjects (n = 13) receiving lamivudine twice daily showed that isolates from 4 of 13 subjects exhibited a 29- to 159-fold decrease in susceptibility to lamivudine, isolates from all 13 subjects were susceptible to zidovudine, and isolates from 3 of 13 subjects exhibited a 21- to 342-fold decrease in susceptibility to efavirenz.
Trial EPV40001: Fifty subjects received lamivudine 300 mg once daily plus zidovudine 300 mg twice daily plus abacavir 300 mg twice daily and 50 subjects received lamivudine 150 mg plus zidovudine 300 mg plus abacavir 300 mg all twice-daily. The median baseline plasma HIV-1 RNA levels for subjects in the 2 groups were 4.79 log10 copies per mL and 4.83 log10 copies per mL, respectively. Fourteen of 50 subjects in the lamivudine once-daily treatment group and 9 of 50 subjects in the lamivudine twice-daily group were identified as virologic failures.
Genotypic analysis of on-therapy HIV-1 isolates from subjects (n = 9) in the lamivudine once-daily treatment group showed that isolates from 6 subjects had an abacavir and/or lamivudine resistance-associated substitution M184V alone. On-therapy isolates from subjects (n = 6) receiving lamivudine twice daily showed that isolates from 2 subjects had M184V alone, and isolates from 2 subjects harbored the M184V substitution in combination with zidovudine resistance-associated amino acid substitutions.
Phenotypic analysis of on-therapy isolates from subjects (n = 6) receiving lamivudine once daily showed that HIV-1 isolates from 4 subjects exhibited a 32- to 53-fold decrease in susceptibility to lamivudine. HIV-1 isolates from these 6 subjects were susceptible to zidovudine.
Phenotypic analysis of on-therapy isolates from subjects (n = 4) receiving lamivudine twice daily showed that HIV-1 isolates from 1 subject exhibited a 45-fold decrease in susceptibility to lamivudine and a 4.5-fold decrease in susceptibility to zidovudine.
Pediatrics: Pediatric subjects receiving lamivudine oral solution concomitantly with other antiretroviral oral solutions (abacavir, nevirapine/efavirenz, or zidovudine) in ARROW developed viral resistance more frequently than those receiving tablets. At randomization to once-daily or twice-daily dosing of lamivudine plus abacavir, 13% of subjects who started on tablets and 32% of subjects who started on solution had resistance substitutions. The resistance profile observed in pediatrics is similar to that observed in adults in terms of the genotypic substitutions detected and relative frequency, with the most commonly detected substitutions at M184 (V or I) [see Clinical Studies (14.2)].
Cross-resistance
Cross-resistance has been observed among nucleoside reverse transcriptase inhibitors (NRTIs). Lamivudine-resistant HIV-1 mutants were cross-resistant in cell culture to didanosine (ddI). Cross-resistance is also expected with abacavir and emtricitabine as these select M184V substitutions.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term carcinogenicity studies with lamivudine in mice and rats showed no evidence of carcinogenic potential at ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies with lamivudine in mice and rats showed no evidence of carcinogenic potential at exposures up to 10 times (mice) and 58 times (rats) the human exposures at the recommended dose of 300 mg.
Mutagenesis
Lamivudine was mutagenic in an L5178Y mouse lymphoma assay and clastogenic in a cytogenetic assay using cultured human lymphocytes. Lamivudine was not mutagenic in a microbial mutagenicity assay, in an in vitro cell transformation assay, in a rat micronucleus test, in a rat bone marrow cytogenetic assay, and in an assay for unscheduled DNA synthesis in rat liver. Lamivudine showed no evidence of in vivo genotoxic activity in the rat at oral doses of up to 2,000 mg per kg, producing plasma levels of 35 to 45 times those in humans at the recommended dose for HIV-1 infection.
Impairment of Fertility
In a study of reproductive performance, lamivudine administered to rats at doses up to 4,000 mg per kg per day, producing plasma levels 47 to 70 times those in humans, revealed no evidence of impaired fertility and no effect on the survival, growth, and development to weaning of the offspring.
-
14 CLINICAL STUDIESThe use of lamivudine are based on the results of clinical trials in HIV-1-infected subjects in combination regimens with other antiretroviral agents. Information from trials with clinical ...
The use of lamivudine are based on the results of clinical trials in HIV-1-infected subjects in combination regimens with other antiretroviral agents. Information from trials with clinical endpoints or a combination of CD4+ cell counts and HIV-1 RNA measurements is included below as documentation of the contribution of lamivudine to a combination regimen in controlled trials.
14.1 Adult Subjects
NUCB3007 (CAESAR) was a multicenter, double-blind, placebo-controlled trial comparing continued current therapy (zidovudine alone [62% of subjects] or zidovudine with didanosine or zalcitabine [38% of subjects]) to the addition of lamivudine or lamivudine plus an investigational non-nucleoside reverse transcriptase inhibitor (NNRTI), randomized 1:2:1. A total of 1,816 HIV-1-infected adults with 25 to 250 CD4+ cells per mm3(median = 122 cells per mm3) at baseline were enrolled: median age was 36 years, 87% were male, 84% were nucleoside-experienced, and 16% were therapy-naive. The median duration on trial was 12 months. Results are summarized in Table 9.
Table 9. Number of Subjects (%) with at Least One HIV-1 Disease Progression Event or Death - *
- An investigational non-nucleoside reverse transcriptase inhibitor not approved in the United States.
Endpoint
Current Therapy
(n = 460)
Lamivudine plus
Current Therapy
(n = 896)
Lamivudine plus an NNRTI* plus Current Therapy
(n = 460)
HIV-1 progression or death
90 (19.6%)
86 (9.6%)
41 (8.9%)
Death
27 (5.9%)
23 (2.6%)
14 (3.0%)
Dual Nucleoside Analogue Trials: Principal clinical trials in the initial development of lamivudine compared lamivudine/zidovudine combinations with zidovudine monotherapy or with zidovudine plus zalcitabine. These trials demonstrated the antiviral effect of lamivudine in a 2-drug combination. More recent uses of lamivudine in treatment of HIV-1 infection incorporate it into multiple-drug regimens containing at least 3 antiretroviral drugs for enhanced viral suppression.
Dose Regimen Comparison Surrogate Endpoint Trials in Therapy-Naive Adults: EPV20001 was a multi-center, double-blind, controlled trial in which subject were randomized 1:1 to receive lamivudine 300 mg once daily or lamivudine 150 mg twice daily, in combination with zidovudine 300 mg twice daily and efavirenz 600 mg once daily. A total of 554 antiretroviral treatment-naive HIV-1-infected adults enrolled: male (79%), white (50%), median age of 35 years, baseline CD4+ cell counts of 69 to 1,089 cells per mm3(median = 362 cells per mm3), and median baseline plasma HIV-1 RNA of 4.66 log10 copies per mL. Outcomes of treatment through 48 weeks are summarized in Figure 1 and Table 10.
Figure 1. Virologic Response through Week 48, EPV20001ab(Intent-to-Treat)
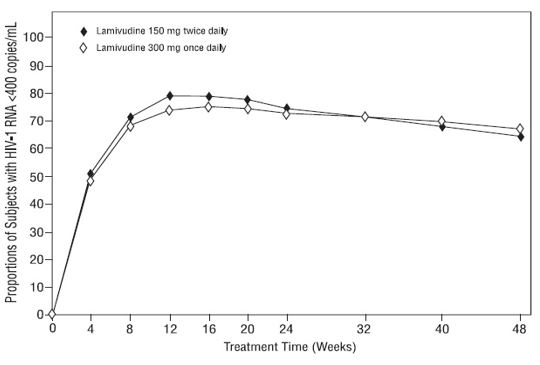
a Roche AMPLICOR HIV-1 MONITOR.
bResponders at each visit are subjects who had achieved and maintained HIV-1 RNA less than 400 copies per mL without discontinuation by that visit.
Table 10. Outcomes of Randomized Treatment through 48 Weeks (Intent-to-Treat) - *
- Achieved confirmed plasma HIV-1 RNA less than 400 copies/mL and maintained through 48 weeks.
- †
- Achieved suppression but rebounded by Week 48, discontinued due to virologic failure, insufficient viral response according to the investigator, or never suppressed through Week 48.
- ‡
- Includes consent withdrawn, lost to follow-up, protocol violation, data outside the trial-defined schedule, and randomized but never initiated treatment.
Outcome
Lamivudine 300 mg Once Daily
plus RETROVIR
plus Efavirenz
(n = 278)
Lamivudine 150 mg Twice Daily plus RETROVIR plus Efavirenz
(n = 276)
Responder*
67%
65%
Virologic failure†
8%
8%
Discontinued due to clinical
<1%
0%
Discontinued due to adverse events
6%
12%
Discontinued due to other reasons‡
18%
14%
The proportions of subjects with HIV-1 RNA less than 50 copies per mL (via Roche Ultrasensitive assay) through Week 48 were 61% for subjects receiving lamivudine tablets 300 mg once daily and 63% for subjects receiving lamivudine tablets 150 mg twice daily. Median increases in CD4+ cell counts were 144 cells per mm3 at Week 48 in subjects receiving lamivudine tablets 300 mg once daily and 146 cells per mm3for subjects receiving lamivudine tablets 150 mg twice daily.
A small, randomized, open-label pilot trial, EPV40001, was conducted in Thailand. A total of 159 treatment-naive adult subjects (male 32%, Asian 100%, median age 30 years, baseline median CD4+ cell count 380 cells per mm3, median plasma HIV-1 RNA 4.8 log10 copies per mL) were enrolled. Two of the treatment arms in this trial provided a comparison between lamivudine 300 mg once daily (n = 54) and lamivudine 150 mg twice daily (n = 52), each in combination with zidovudine 300 mg twice daily and abacavir 300 mg twice daily. In intent-to-treat analyses of 48-week data, the proportions of subjects with HIV-1 RNA below 400 copies per mL were 61% (33 of 54) in the group randomized to once-daily lamivudine and 75% (39 of 52) in the group randomized to receive all 3 drugs twice daily; the proportions with HIV-1 RNA below 50 copies per mL were 54% (29 of 54) in the once-daily lamivudine group and 67% (35 of 52) in the all-twice-daily group; and the median increases in CD4+ cell counts were 166 cells per mm3 in the once-daily lamivudine group and 216 cells per mm3 in the all-twice-daily group.
Close14.2 Pediatric Subjects
ACTG300 was a multicenter, randomized, double-blind trial that provided for comparison of lamivudine plus RETROVIR (zidovudine) with didanosine monotherapy. A total of 471 symptomatic, HIV-1-infected therapy-naive (less than or equal to 56 days of antiretroviral therapy) pediatric subjects were enrolled in these 2 treatment arms. The median age was 2.7 years (range: 6 weeks to 14 years), 58% were female, and 86% were non-white. The mean baseline CD4+ cell count was 868 cells per mm3 (mean: 1,060 cells per mm3 and range: 0 to 4,650 cells per mm3 for subjects aged less than or equal to 5 years; mean: 419 cells per mm3 and range: 0 to 1,555 cells per mm3 for subjects aged over 5 years) and the mean baseline plasma HIV-1 RNA was 5.0 log10 copies per mL. The median duration on trial was 10.1 months for the subjects receiving lamivudine plus RETROVIR and 9.2 months for subjects receiving didanosine monotherapy. Results are summarized in Table 11.
Table 11. Number of Subjects (%) Reaching a Primary Clinical Endpoint (Disease Progression or Death) Endpoint
Lamivudine plus RETROVIR
(n = 236)
Didanosine
(n = 235)
HIV-1 disease progression or death (total)
15 (6.4%)
37 (15.7%)
Physical growth failure
7 (3.0%)
6 (2.6%)
Central nervous system deterioration
4 (1.7%)
12 (5.1%)
CDC Clinical Category C
2 (0.8%)
8 (3.4%)
Death
2 (0.8%)
11 (4.7%)
ARROW (COL105677) was a 5-year randomized, multicenter trial which evaluated multiple aspects of clinical management of HIV-1 infection in pediatric subjects. HIV-1-infected, treatment-naïve subjects aged 3 months to 17 years were enrolled and treated with a first-line regimen containing lamivudine and abacavir, dosed twice daily according to World Health Organization recommendations. After a minimum of 36 weeks on treatment, subjects were given the option to participate in Randomization 3 of the ARROW trial, comparing the safety and efficacy of once-daily dosing with twice-daily dosing of lamivudine and abacavir, in combination with a third antiretroviral drug, for an additional 96 weeks. Of the 1,206 original ARROW subjects, 669 participated in Randomization 3. Virologic suppression was not a requirement for participation: at baseline for Randomization 3 (following a minimum of 36 weeks of twice-daily treatment), 75% of subjects in the twice-daily cohort were virologically suppressed, compared with 71% of subjects in the once-daily cohort.
The proportion of subjects with HIV-1 RNA of less than 80 copies per mL through 96 weeks is shown in Table 12. The differences between virologic responses in the two treatment arms were comparable across baseline characteristics for gender and age.
Table 12. Virologic Outcome of Randomized Treatment at Week 96a (ARROW Randomization 3) aAnalyses were based on the last observed viral load data within the Week 96 window.
bPredicted difference (95% CI) of response rate is -4.5% (-11% to 2%) at Week 96.
cIncludes subjects who discontinued due to lack or loss of efficacy or for reasons other than an adverse event or death, and had a viral load value of greater than or equal to 80 copies per mL, or subjects who had a switch in background regimen that was not permitted by the protocol.
dOther includes reasons such as withdrew consent, loss to follow-up, etc. and the last available HIV-1 RNA less than 80 copies per mL (or missing).
Outcome
Lamivudine plus Abacavir Twice-Daily Dosing (n = 333)
Lamivudine plus Abacavir Once-Daily Dosing (n = 336)
HIV-1 RNA <80 copies/mLb
70%
67%
HIV-1 RNA ≥80 copies/mLc
No virologic data
28%
31%
Discontinued due to adverse event or death
1%
<1%
Discontinued study for other reasonsd
0%
<1%
Missing data during window but on study
1%
1%
Analyses by formulation demonstrated the proportion of subjects with HIV-1 RNA of less than 80 copies per mL at randomization and Week 96 was higher in subjects who had received tablet formulations of lamivudine and abacavir (75% [458/610] and 72% [434/601]) than in those who had received solution formulation(s) (with Lamivudine oral solution given at weight band-based doses approximating 8 mg per kg per day) at any time (52% [29/56] and 54% [30/56]), respectively [see Warnings and Precautions (5.5)]. These differences were observed in each different age group evaluated.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLamivudine Tablets USP, 150 mg - Scored - White, circular, biconvex, film coated tablets 'TMT' engraved on one side and breakline on other side. Bottle of 60 tablets (NDC 64380-710-03) with ...
Lamivudine Tablets USP, 150 mg - Scored
White, circular, biconvex, film coated tablets 'TMT' engraved on one side and breakline on other side.
Bottle of 60 tablets (NDC 64380-710-03) with child-resistant closure.
Lamivudine Tablets USP, 300 mg
White, circular, biconvex, film coated tablets "L300" engraved on one side and plain on other side.
Bottle of 30 tablets (NDC 64380-711-04) with child-resistant closure.
Recommended Storage:
Store lamivudine tablets at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Patients with Hepatitis B or C Co-infection - Inform patients co-infected with HIV-1 and HBV that ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients with Hepatitis B or C Co-infection
Inform patients co-infected with HIV-1 and HBV that deterioration of liver disease has occurred in some cases when treatment with lamivudine was discontinued. Advise patients to discuss any changes in regimen with their healthcare provider [see Warnings and Precautions (5.1)].
Differences in Formulations of Lamivudine
Advise patients that Lamivudine tablets contain a higher dose of the same active ingredient (lamivudine) as EPIVIR-HBV tablets and oral solution. If a decision is made to include lamivudine in the HIV-1 treatment regimen of a patient co-infected with HIV-1 and HBV, the formulation and dosage of lamivudine in Lamivudine tablets (not EPIVIR-HBV) should be used [see Warnings and Precautions (5.1)].
Lactic Acidosis/Hepatomegaly with Steatosis
Advise patients that lactic acidosis and severe hepatomegaly with steatosis have been reported with use of nucleoside analogues and other antiretrovirals. Advise patients to stop taking Lamivudine Tablets if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.2)].
Risk of Pancreatitis
Advise parents or guardians to monitor pediatric patients for signs and symptoms of pancreatitis [see Warnings and Precautions (5.3)].
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any signs and symptoms of infection as inflammation from previous infection may occur soon after combination antiretroviral therapy, including when lamivudine tablets is started [see Warnings and Precautions (5.4)].
Lower Virologic Suppression Rates and Increased Risk of Viral Resistance with Oral Solution
Advise patients that an all-tablet regimen should be used when possible due to an increased rate of treatment failure among pediatric subjects who received Lamivudine oral solution concomitantly with other antiretroviral oral solutions [see Warnings and Precautions (5.5)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to lamivudine tablets during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk [see Use in Specific Populations (8.2)].
Missed Dosage
Instruct patients that if they miss a dose of lamivudine tablets to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose [see Dosage and Administration (2)].
EPIVIR, RETROVIR and EPIVIR-HBV are registered trademarks of their respective owners and are not trademarks of Strides Pharma Science Limited. The makers of these brands are not affiliated with and do not endorse Strides Pharma Science Limited or its products.
Manufactured by:
Strides Pharma Science Ltd.
Bengaluru - 562106, India
Distributed by:
Strides Pharma Inc.
East Brunswick, NJ 08816
Revised: 11/2019
PHARMACIST-DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
Close -
PATIENT INFORMATIONLamivudine tablets, USP - (la miv' ue deen) What is the most important information I should know about lamivudine tablets? Lamivudine tablets can cause serious side effects ...
(la miv' ue deen)
What is the most important information I should know about lamivudine tablets?
Lamivudine tablets can cause serious side effects, including:
-
Worsening of hepatitis B virus in people who have HIV-1 infection. If you have HIV-1 (Human Immunodeficiency Virus type 1) and hepatitis B virus (HBV) infection, your HBV may get worse (flare-up) if you stop taking lamivudine tablet. A "flare-up" is when your HBV infection suddenly returns in a worse way than before. Worsening liver disease can be serious and may lead to death.
- Do not run out of lamivudine tablets. Refill your prescription or talk to your healthcare provider before your lamivudine tablets is all gone.
- Do not stop lamivudine tablets without first talking to your healthcare provider.
- If you stop taking lamivudine tablets, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your liver.
Lamivudine tablets is a prescription medicine used together with other antiretroviral medicines to treat Human Immunodeficiency Virus (HIV-1) infection.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
Lamivudine tablets (used to treat HIV-1 infection) contain a higher dose of the same active ingredient (lamivudine) than is in the medicine EPIVIR-HBV tablets and oral solution (used to treat HBV). If you have both HIV-1 and HBV, you should not use EPIVIR-HBV to treat your infections.
The safety and effectiveness of lamivudine tablets have not been established in children under 3 months of age.
Who should not take lamivudine tablets?
Do not take lamivudine tablets if you are allergic to lamivudine or any of the ingredients in lamivudine tablets. See the end of this Patient Information leaflet for a complete list of ingredients in lamivudine tablets.
What should I tell my healthcare provider before taking lamivudine tablets?
Before you take lamivudine tablets, tell your healthcare provider if you:
- have or have had liver problems, including hepatitis B or C virus infection.
- have kidney problems.
- are pregnant or plan to become pregnant. Taking lamivudine tablets during pregnancy has not been associated with an increased risk of birth defects. Talk to your healthcare provider if you are pregnant or plan to become pregnant
Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take lamivudine tablets.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medications interact with lamivudine tablets. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. You can ask your healthcare provider or pharmacist for a list of medicines that interact with lamivudine tablets.
Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take lamivudine tablets with other medicines.
How should I take lamivudine tablets?
- Take lamivudine tablets exactly as your healthcare provider tells you to take it.
- If you miss a dose of lamivudine tablets, take it as soon as you remember. Do not take 2 doses at the same time or take more than what your healthcare provider tells you to take.
- Stay under the care of a healthcare provider during treatment with lamivudine tablets.
- Lamivudine tablets may be taken with or without food.
- For children 3 months and older, your healthcare provider will prescribe a dose of lamivudine tablets based on your child's body weight.
- Tell your healthcare provider if you or your child has trouble swallowing tablets.
- Do not run out of lamivudine tablets. The virus in your blood may increase and the virus may become harder to treat. When your supply starts to run low, get more from your healthcare provider or pharmacy.
- If you take too much lamivudine tablets, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of lamivudine tablets?
- Lamivudine tablets cancause serious side effects including:
- See "What is the most important information I should know about lamivudine tablets?"
-
Build-up of an acid in your blood (lactic acidosis). Lactic acidosis can happen in some people who take lamivudine tablets. Lactic acidosis is a serious medical emergency that can cause death. Call your healthcare provider right away if you get any of the following symptoms that could be signs of lactic acidosis:
- feel very weak or tired
- feel cold, especially in your arms and legs
- unusual (not normal) muscle pain
- feel dizzy or light-headed
- trouble breathing
- have a fast or irregular heartbeat
- stomach pain with nausea and vomiting
-
Serious liver problems can happen in people who take lamivudine tablets. In some cases these serious liver problems can lead to death. Your liver may become large (hepatomegaly) and you may develop fat in your liver (steatosis). Call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:
- your skin or the white part of your eyes turns yellow (jaundice)
- loss of appetite for several days or longer
- nausea
- dark or "tea-colored" urine
- pain, aching, or tenderness on the right side of your stomach area
- light-colored stools (bowel movements)
You may be more likely to get lactic acidosis or serious liver problems if you are female or very overweight (obese).
-
Risk of inflammation of the pancreas (pancreatitis). Children may be at risk for developing pancreatitis during treatment with lamivudine tablets if they:
- have taken nucleoside analogue medicines in the past
- have a history of pancreatitis
- have other risk factors for pancreatitis
Call your healthcare provider right away if your child develops signs and symptoms of pancreatitis including severe upper stomach-area pain, with or without nausea and vomiting. Your healthcare provider may tell you to stop giving lamivudine tablets to your child if their symptoms and blood test results show that your child may have pancreatitis.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after you start taking lamivudine tablets.
The most common side effects of lamivudine tablets in adults include:
- headache
- nasal signs and symptoms
- nausea
- diarrhea
- generally not feeling well
- tiredness
- cough
The most common side effects of lamivudine tablets in children include fever and cough.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of lamivudine tablets. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lamivudine tablets?
- Store lamivudine tablets at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Keep lamivudine tablets and all medicines out of the reach of children
General information about the safe and effective use of lamivudine tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use lamivudine tablets for a condition for which it was not prescribed. Do not give lamivudine tablets to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about lamivudine tablets that is written for health professionals.
You may report side effects to Strides Pharma Inc. at 1-877-244-9825 or go to www.strides.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
What are the ingredients in lamivudine tablets?
Active ingredient: lamivudine
Inactive ingredients:
Lamivudine scored 150-mg film-coated tablets: microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, povidone, magnesium stearate, and opadry white which is composed of Hydroxy Propyl methylcellulose 2910/ Hypromellose 5cP, Titanium dioxide, Polyethylene glycol 400 (Macrogol).
Lamivudine 300-mg film-coated tablets: microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, povidone, magnesium stearate, and opadry white which is composed of Hydroxy Propyl methylcellulose 2910/ Hypromellose 5cP, Titanium dioxide, Polyethylene glycol 400 (Macrogol).
This Patient Information has been approved by the U.S. Food and Drug Administration.
EPIVIR and EPIVIR-HBV are registered trademarks of their respective owners and are not trademarks of Strides Pharma Science Limited. The makers of these brands are not affiliated with and do not endorse Strides Pharma Science Limited or its products.
Manufactured by:
Strides Pharma Science Ltd.
Bengaluru – 562106, India
Distributed by:
Strides Pharma Inc.
East Brunswick, NJ 08816
Revision: 11/2019
Close -
Worsening of hepatitis B virus in people who have HIV-1 infection. If you have HIV-1 (Human Immunodeficiency Virus type 1) and hepatitis B virus (HBV) infection, your HBV may get worse (flare-up) if you stop taking lamivudine tablet. A "flare-up" is when your HBV infection suddenly returns in a worse way than before. Worsening liver disease can be serious and may lead to death.
-
PRINCIPAL DISPLAY PANELNDC 64380-710-03 - Lamivudine Tablets USP - 150 mg - Rx only - 60 Tablets - Each tablet contains 150 mg of lamivudine. See prescribing information for dosage information. Store lamivudine tablets ...
Lamivudine Tablets USP
150 mg
Rx only
60 Tablets
Each tablet contains 150 mg of lamivudine.
See prescribing information for dosage information.
Store lamivudine tablets at 20° to 25°C (68° to 77°F)
[see USP Controlled Room Temperature]. Protect from light.
Manufactured by:
Strides Pharma Science Limited
Bengaluru-562106, India
Distributed by:
Strides Pharma Inc.
East Brunswick, NJ 08816
Revision: 11/2019
Lamivudine Tablets USP
300 mg
R only
30 Tablets
Each tablet contains 150 mg of lamivudine.
See prescribing information for dosage information.
Store lamivudine tablets at 20° to 25°C (68° to 77°F)
[see USP Controlled Room Temperature]. Protect from light.
Manufactured by:
Strides Pharma Science Limited
Bengaluru-562106, India
Distributed by:
Strides Pharma Inc.
East Brunswick, NJ 08816
Revision: 11/2019
Close
-
INGREDIENTS AND APPEARANCEProduct Information
LAMIVUDINE lamivudine tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64380-710 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAMIVUDINE (UNII: 2T8Q726O95) (LAMIVUDINE - UNII:2T8Q726O95) LAMIVUDINE 150 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) Product Characteristics Color WHITE Score no score Shape ROUND (circular biconvex) Size 9mm Flavor Imprint Code TMT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64380-710-03 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090457 10/19/2018 LAMIVUDINE lamivudine tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64380-711 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAMIVUDINE (UNII: 2T8Q726O95) (LAMIVUDINE - UNII:2T8Q726O95) LAMIVUDINE 300 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) Product Characteristics Color WHITE Score no score Shape ROUND (circular biconvex) Size 12mm Flavor Imprint Code L300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64380-711-04 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090457 10/19/2018 Labeler - Strides Pharma Science Limited (650738743) Registrant - Strides Pharma Science Limited (650738743)
CloseEstablishment Name Address ID/FEI Business Operations Strides Pharma Science Limited 918513263 ANALYSIS(64380-710, 64380-711) , MANUFACTURE(64380-710, 64380-711) , PACK(64380-710, 64380-711)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LAMIVUDINE tablet, film coated
Number of versions: 7
RxNorm
LAMIVUDINE tablet, film coated
Get Label RSS Feed for this Drug
LAMIVUDINE tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=ac824c7e-9c9f-487b-ad2f-d50eaef5cbda
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LAMIVUDINE tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 64380-710-03 |
| 2 | 64380-711-04 |