Label: AZITHROMYCIN tablet, film coated
- NDC Code(s): 50111-789-10
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AZITHROMYCIN TABLETS safely and effectively. See full prescribing information for AZITHROMYCIN TABLETS. AZITHROMYCIN tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAzithromycin tablets are a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in ...
-
2 DOSAGE AND ADMINISTRATION[see Indications and Usage (1)] Not for pediatric use. For pediatric patients, please refer to the INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION sections of the prescribing information ...
-
3 DOSAGE FORMS AND STRENGTHSAzithromycin tablets USP, 600 mg are supplied as white, capsule shaped, unscored, biconvex film-coated tablets, debossed with “789” on one side and “PLIVA” on the other, containing azithromycin ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Azithromycin tablets are contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide, or ketolide drug. 4.2 Hepatic ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Acute Generalized Exanthematous Pustulosis (AGEP), Stevens-Johnson ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Hypersensitivity [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Nelfinavir - Coadministration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any ...
-
10 OVERDOSAGEAdverse reactions experienced in higher than recommended doses were similar to those seen at normal doses. In the event of overdosage, general symptomatic and supportive measures are indicated as ...
-
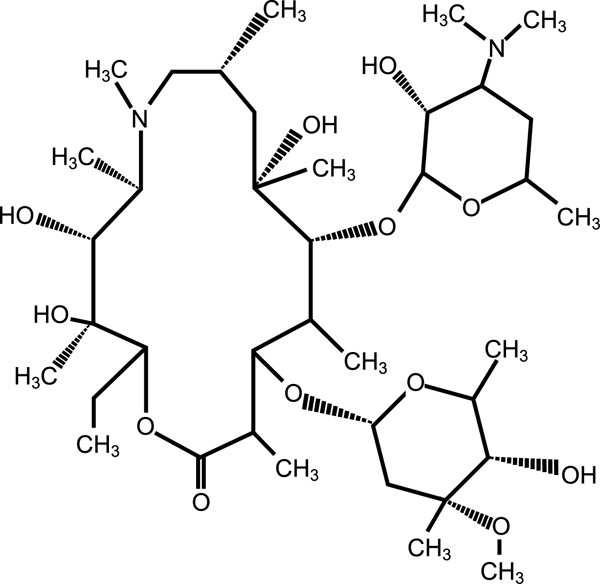
11 DESCRIPTIONAzithromycin tablets USP, contain the active ingredient azithromycin, USP, a macrolide antibacterial drug, for oral administration. Azithromycin, USP has the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Azithromycin is a macrolide antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Based on animal models of infection, the antibacterial activity of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential ...
-
14 CLINICAL STUDIES14.1 Clinical Studies in Patients with Advanced HIV Infection for the Prevention and Treatment of Disease Due to Disseminated Mycobacterium avium Complex (MAC) [see Indications and Usage ...
-
15 REFERENCESGriffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAzithromycin tablets USP, 600 mg are supplied as white, capsule shaped, unscored, biconvex film-coated tablets, debossed with “789” on one side and “PLIVA” on the other, containing azithromycin ...
-
17 PATIENT COUNSELING INFORMATIONAzithromycin tablets may be taken with or without food. However, increased tolerability has been observed when tablets are taken with food. Patients should also be cautioned not to take ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 50111-789-10 - Azithromycin Tablets USP - 600 mg* Rx only - 30 TABLETS
-
INGREDIENTS AND APPEARANCEProduct Information