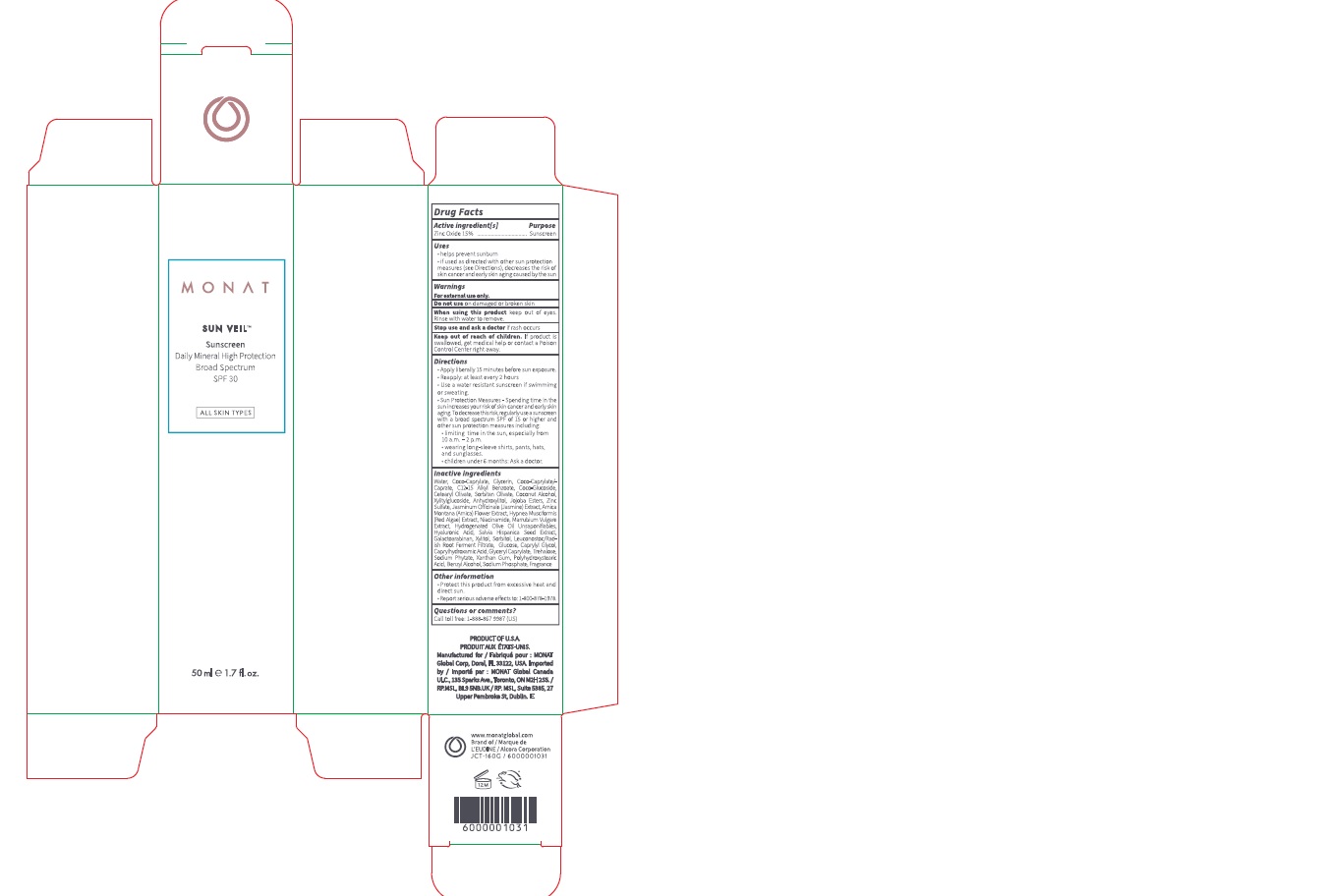
Label: MONAT SUN VEIL SUNSCREEN BROAD SPECTRUM SPF 30- zinc oxide lotion
- NDC Code(s): 78518-001-01
- Packager: Monat Global Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient[s]
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply: at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures - Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limiting time in the sun, especially from 10 a.m. - 2 p.m.
- wearing long-sleeve shirts, pants, hats, and sunglasses.
- children under 6 months: Ask a doctor.
-
Inactive ingredients
Water, Coco-Caprylate, Glycerin, Coco-Caprylate/Caprate, C12-15 Alkyl Benzoate, Coco-Glucoside, Cetearyl Olivate, Soibitan Olivate, Coconut Alcohol, Xylitylgucoside, Anhydroxylitol, Jojoba Esters, Zinc Sulfate, Jasminum Officinale (Jasmine) Extract, Arnica Montana (Arnica) Rower Extract, Hypnea Musciformis (Red Algae) Extract, Niacinamide, Marrubium Vulgare Extract, Hydrogenated Olive Oil Unsaponifiables, Hyaluronic Acid, Salvia Hispanica Seed Extract, Galactoarabinan, Xylitol, Sorbitol, Leuconostoc/Rad- ish Root Ferment Filtrate, Glucose, Caprylyl Glycol, CaprylhydroxamicAcid, Glyceryl Caprylate, Trehalose, Sodium Phytate, Xanthan Gum, Polyhydroxystearic Add, Benzyl Alcohol, Sodium Phosphate, Fragrance
- Other information
- Questions or comments?
- Company Information
- Product Packaging
-
INGREDIENTS AND APPEARANCE
MONAT SUN VEIL SUNSCREEN BROAD SPECTRUM SPF 30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78518-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HOREHOUND (UNII: K08036XEJV) HYALURONIC ACID (UNII: S270N0TRQY) CHIA SEED (UNII: NU0OLX06F8) XANTHAN GUM (UNII: TTV12P4NEE) BENZYL ALCOHOL (UNII: LKG8494WBH) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARYL OLIVATE (UNII: 58B69Q84JO) COCONUT ALCOHOL (UNII: 13F4MW8Y9K) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) ANHYDROXYLITOL (UNII: 8XWR7NN42F) COCO-CAPRYLATE (UNII: 4828G836N6) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) COCO GLUCOSIDE (UNII: ICS790225B) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) TREHALOSE (UNII: B8WCK70T7I) SORBITAN OLIVATE (UNII: MDL271E3GR) XYLITOL (UNII: VCQ006KQ1E) SORBITOL (UNII: 506T60A25R) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) PHYTATE SODIUM (UNII: 88496G1ERL) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) WATER (UNII: 059QF0KO0R) ZINC SULFATE (UNII: 89DS0H96TB) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) HYPNEA MUSCIFORMIS (UNII: W6FF9R1FJV) NIACINAMIDE (UNII: 25X51I8RD4) HYDROGENATED OLIVE OIL UNSAPONIFIABLES (UNII: B8MIX97W95) GALACTOARABINAN (UNII: SL4SX1O487) SODIUM PHOSPHATE (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78518-001-01 1 in 1 CARTON 08/03/2020 10/31/2024 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/03/2020 10/31/2024 Labeler - Monat Global Corp. (027036949)