Label: CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE- clindamycin phosphate and benzoyl...view full title
- NDC Code(s): 72162-2299-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 21922-022
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% safely and effectively. See full prescribing information for Clindamycin ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% is indicated for the topical treatment of inflammatory acne vulgaris in patients 12 years and older. 1.2 Limitations ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% to the face once daily, in the evening or as directed by the physician. The skin should be gently washed, rinsed with ...
-
3 DOSAGE FORMS AND STRENGTHSGel, 1.2%/5% Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% is a white to slightly yellow, opaque gel. Each gram of Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% contains 12 mg ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% is contraindicated in those individuals who have shown hypersensitivity to clindamycin, benzoyl peroxide, any ...
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated following topical use of clindamycin. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been ...
-
6 ADVERSE REACTIONSThe following adverse reaction is described in more detail in the - Warnings and Precautions section of the label: Colitis - [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Erythromycin - Avoid using Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% in combination with erythromycin-containing products due to its clindamycin component. In vitro studies have ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women treated with Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5%. Clindamycin ...
-
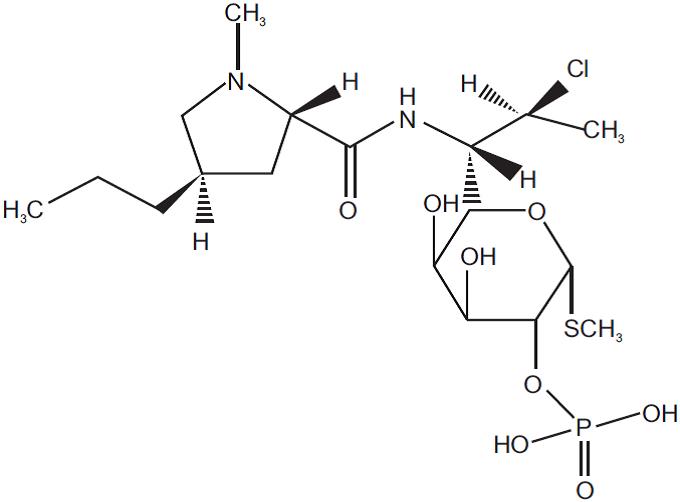
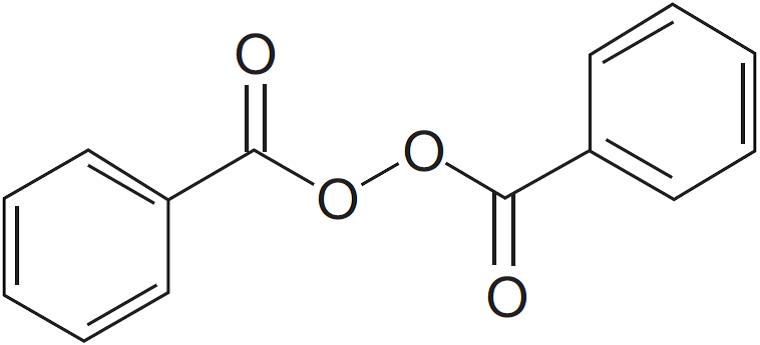
11 DESCRIPTIONClindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% is a fixed combination product with two active ingredients in a white to slightly yellow, opaque, aqueous gel formulation. Clindamycin ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clindamycin - Clindamycin is a lincosamide antibacterial - [see Clinical Pharmacology ( 12.4)]. Benzoyl Peroxide - Benzoyl ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility - Benzoyl peroxide has been shown to be a tumor promoter and progression agent in a number of animal studies. Benzoyl peroxide in acetone ...
-
14 CLINICAL STUDIESIn five randomized, double-blind clinical trials of 1,319 subjects, 397 used Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5%, 396 used benzoyl peroxide, 349 used clindamycin, and 177 used ...
-
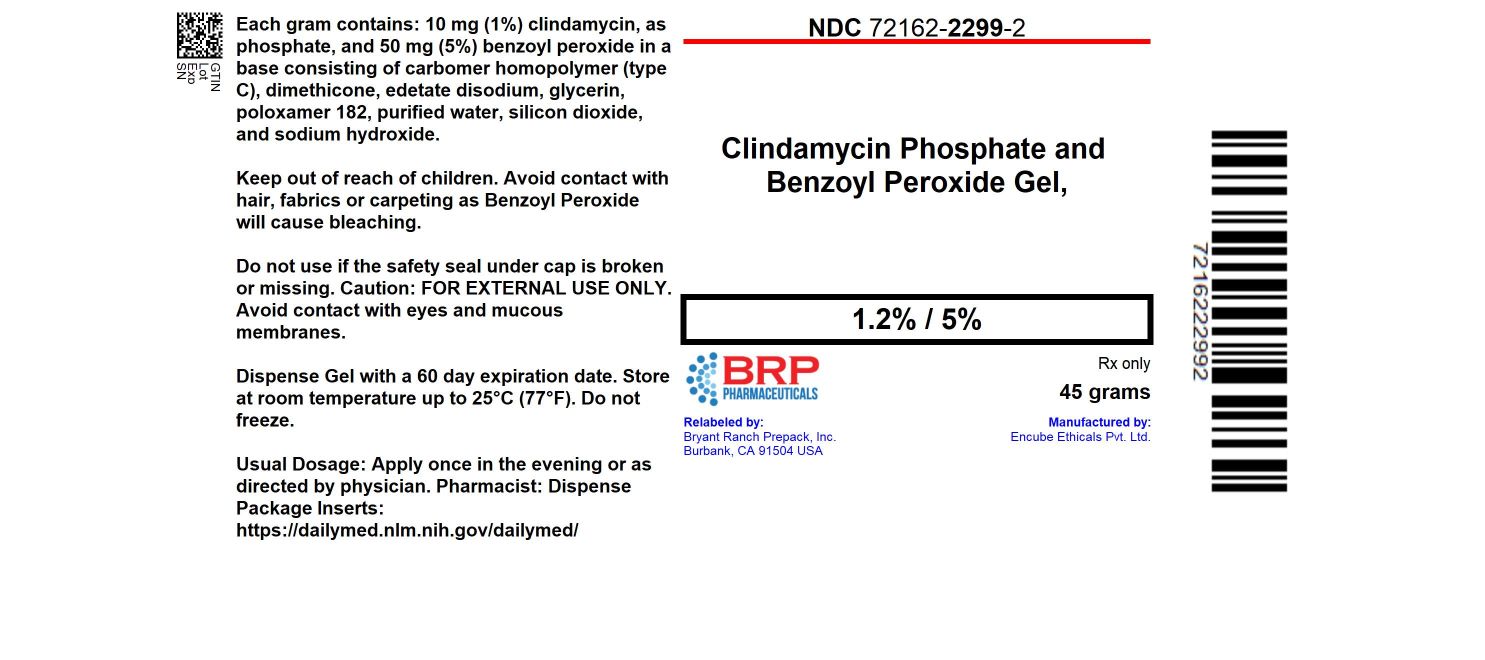
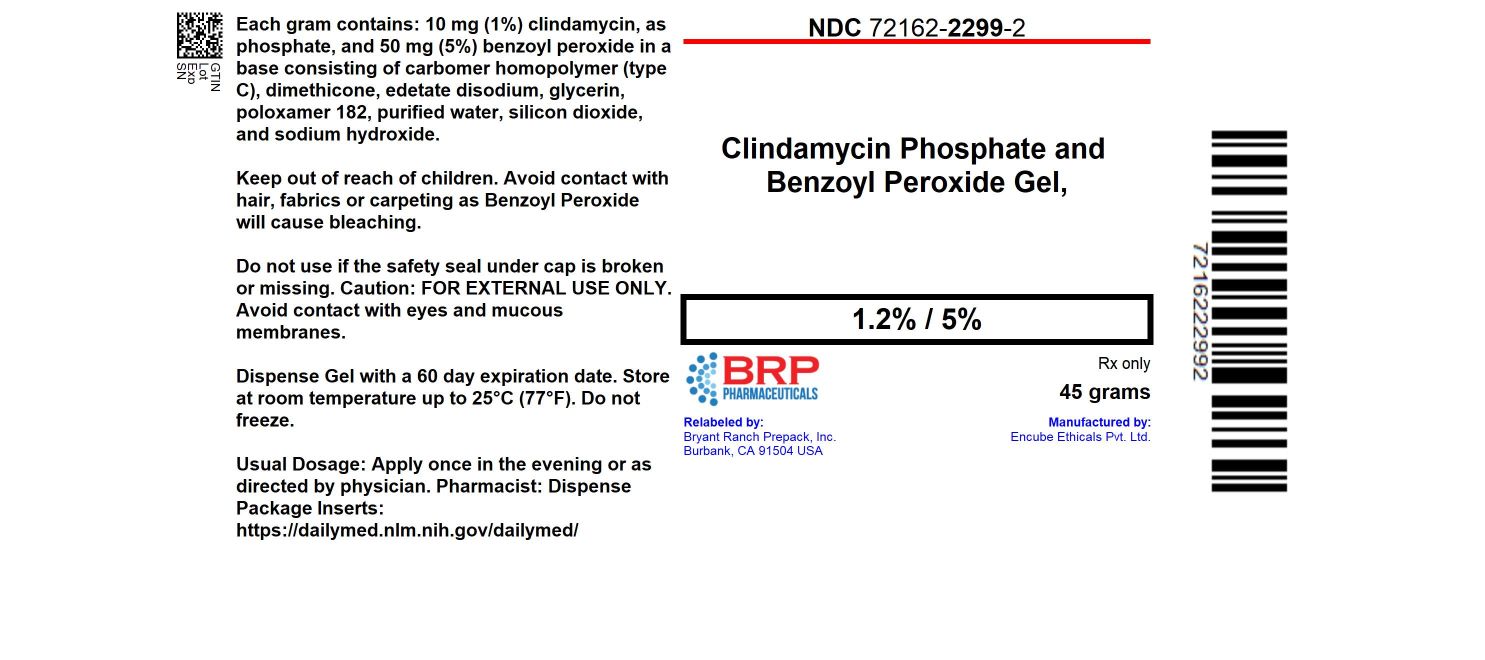
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% is a white to slightly yellow, opaque gel. NDC: 72162-2299-2: 45 g in a TUBE - 16.2 Storage and Handling - Pharmacist: Prior ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Patients who develop allergic reactions such as severe swelling or shortness of breath should discontinue use ...
-
PATIENT INFORMATIONPHARMACIST – DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT - PATIENT INFORMATION - Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% Important: For use on the skin only (topical use) ...
-
PRINCIPAL DISPLAY PANELClindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% Extended Label
-
INGREDIENTS AND APPEARANCEProduct Information