Label: COLISTIMETHATE- colistimethate sodium injection, powder, lyophilized, for solution
- NDC Code(s): 63323-393-06
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Colistimethate for Injection, USP and other antibacterial drugs, Colistimethate for Injection, USP ...
-
DESCRIPTIONColistimethate for Injection, USP is a sterile parenteral antibiotic product which, when reconstituted (see Reconstitution), is suitable for intramuscular or intravenous administration. The color ...
-
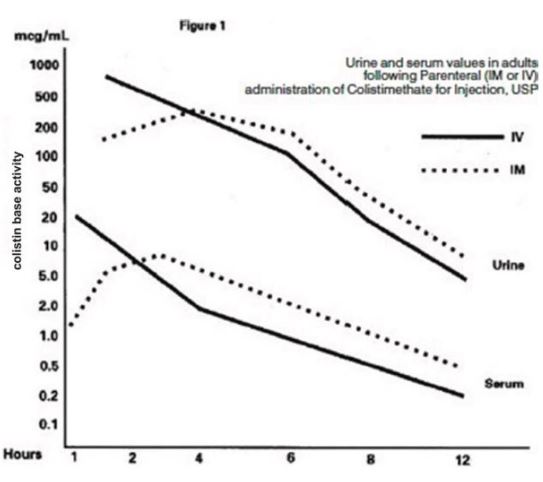
CLINICAL PHARMACOLOGYTypical serum and urine levels following a single 150 mg dose of colistimethate IM or IV in normal adult subjects are shown in Figure 1. Higher serum levels were obtained at 10 minutes ...
-
INDICATIONS AND USAGEColistimethate for Injection, USP is indicated for the treatment of acute or chronic infections due to sensitive strains of certain gram-negative bacilli. It is particularly indicated when the ...
-
CONTRAINDICATIONSThe use of Colistimethate for Injection, USP is contraindicated for patients with a history of sensitivity to the drug or any of its components.
-
WARNINGSMaximum daily dose calculated from colistin base activity should not exceed 5 mg/kg/day with normal renal function. Transient neurological disturbances may occur. These include circumoral ...
-
PRECAUTIONSGeneral - Since colistimethate is eliminated mainly by renal excretion, it should be used with caution when the possibility of impaired renal function exists. The decline in renal function with ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported: Gastrointestinal - gastrointestinal upset - Nervous System - tingling of extremities and tongue, slurred speech, dizziness ...
-
OVERDOSAGEOverdosage with colistimethate sodium can cause neuromuscular blockade characterized by paresthesia, lethargy, confusion, dizziness, ataxia, nystagmus, disorders of speech and apnea. Respiratory ...
-
DOSAGE AND ADMINISTRATIONImportant - Colistimethate for Injection, USP is supplied in vials containing colistimethate sodium equivalent to 150 mg colistin base activity per vial. Reconstitution for Intravenous or ...
-
INTRAVENOUS ADMINISTRATION1. Direct Intermittent Administration - Slowly inject one-half of the total daily dose over a period of 3 to 5 minutes every 12 hours. 2. Continuous Infusion - Slowly inject one-half of the ...
-
HOW SUPPLIEDColistimethate for Injection, USP is supplied in vials containing colistimethate sodium (equivalent to 150 mg colistin base activity per vial) as a white to slightly yellow lyophilized cake ...
-
SPL UNCLASSIFIED SECTIONwww.fresenius-kabi.com/us - 451075D - Revised: January 2020
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY - Colistimethate 150 mg Vial Label - Colistimethate - for Injection, USP - 150 mg* per vial - Colistin base activity - For intramuscular - and intravenous use ...
-
INGREDIENTS AND APPEARANCEProduct Information