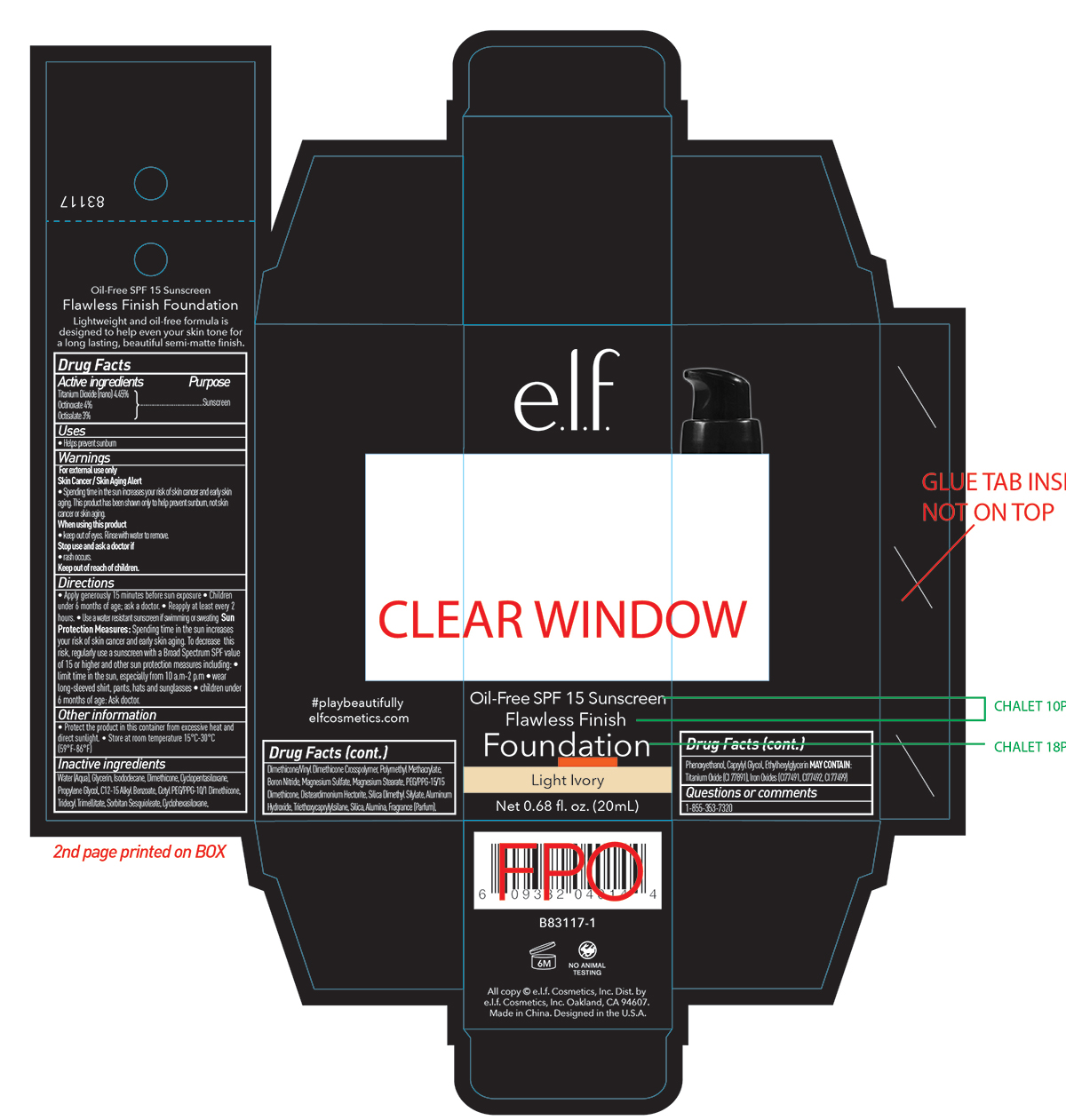
Label: ELF FLAWLESS FINISH FOUNDATION OIL FREE SPF 15 SUNSCREEN LIGHT IVORY- titanium dioxide cream
- NDC Code(s): 76354-631-01
- Packager: e.l.f. Cosmetics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
For suncreen use:
Apply generally 15 min before sun exposure.
Children under six months of age: ask a doctor
Reapply at least every two hours.
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures:
Spending time in sun increases your risk of skin cancer or early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF value of 15 or higher and other sun protection measures including
Other Information:
Protect this product in the container from excessive heat and direct sun
Store at room temperature 15C-30C (59F-86F)
- Limit time in the sun, especially from 10 am to 2 pm
- Wear long sleeved shirts, pants, hats, and sunglasses.
- Children under six months of age: ask a doctor
-
INACTIVE INGREDIENT
Inactive Ingredient:
Water (Aqua), Glycerin, Isododecane, Dimethicone, Cyclopentasiloxane, Propylene Glycol, C12-15 Alkyl Benzoate, Cetyl PEG/PPG-10/1 Dimethicone, Tridecyl Trimellitate, Sorbitan Sesquioleate, Cyclohexasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Polymethyl Methacrylate, Boron Nitride, Magnesium Sulfate, Magnesium Stearate, PEG/PPG-15/15 Dimethicone, Disteardimonium Hectorite, Silica Dimethyl Silylate, Aluminum Hydroxide, Triethoxycaprylylsilane, Silica, Alumina, Fragrance (Parfum), Phenoxyethanol, Caprylyl Glycol, EthylhexylglycerinMA
May contain:
Iron Oxides (CI 77491, CI 77492, CI 77499)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ELF FLAWLESS FINISH FOUNDATION OIL FREE SPF 15 SUNSCREEN LIGHT IVORY
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76354-631 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.45 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) TRIEPOXYCYCLOHEXASILANE (UNII: 066Q83563R) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) PEG/PPG-15/15 DIMETHICONE (UNII: 184IZ28A2G) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MAGNESIUM STEARATE (UNII: 70097M6I30) WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76354-631-01 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/26/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/26/2017 12/12/2024 Labeler - e.l.f. Cosmetics, Inc (093902816) Establishment Name Address ID/FEI Business Operations Zhejiang Ayan Biotech Co., Ltd. 544377996 manufacture(76354-631)