Label: DELFLEX- dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solution
- NDC Code(s): 46163-206-92, 46163-206-94, 46163-206-95, 46163-209-92, view more
- Packager: Fresenius Medical Care de Mexico, S.A. de C.V.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DELFLEX - ®safely and effectively. See full prescribing information for DELFLEX - ®peritoneal dialysis solution with attached ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGEDELFLEX® is indicated in the treatment of chronic kidney failure patients being maintained on peritoneal dialysis
-
2. DOSAGE AND ADMINISTRATION2.1 Basic Dosing Information - DELFLEX® is intended for intraperitoneal administration only. Not for intravenous or intra-arterial administration. The mode of therapy, frequency of treatment ...
-
3. Dosage Forms and StrengthsDELFLEX peritoneal dialysis solutions are available in transparent single-dose flexible bags. The solution is clear with color being slightly yellow to colorless. All DELFLEX peritoneal dialysis ...
-
4. ContraindicationsNone.
-
5. Warnings and Precautions5.1 Electrolyte, Fluid and Nutrition Imbalances - Peritoneal dialysis may affect a patient's protein, water-soluble vitamin, potassium, sodium, chloride, bicarbonate, and magnesium levels and ...
-
6. ADVERSE REACTIONSSolution related adverse reactions may include peritonitis, catheter site infection, electrolyte and fluid imbalances, hypovolemia, hypervolemia, hypertension, hypotension, disequilibrium ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - DELFLEX solutions consist of electrolytes, lactate, and bicarbonate at physiological levels, and glucose to facilitate ultrafiltration. While there are no adequate ...
-
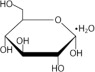
11. DESCRIPTIONThe DELFLEX® peritoneal dialysis solutions (low magnesium/low calcium) are sterile, non-pyrogenic formulations of dextrose and electrolytes in water for injection, USP, for use in peritoneal ...
-
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action - DELFLEX peritoneal dialysis solutions are hypertonic peritoneal dialysis solutions containing dextrose, a monosaccharide, as the primary osmotic agent. An osmotic ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long term animal studies with DELFLEX peritoneal dialysis solutions have not been performed to evaluate the carcinogenic potential ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGDELFLEX peritoneal dialysis solutions with attached stay•safe exchange set are available in the sizes and formulations shown in - Table 1[Dosage Forms and Strengths]. (3) DELFLEX peritoneal ...
-
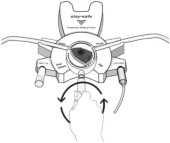
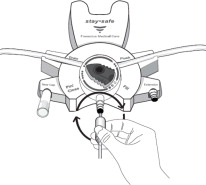
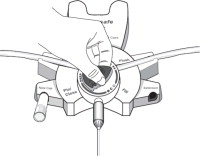
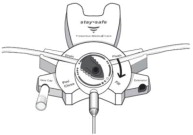
17. PATIENT COUNSELING INFORMATIONAseptic technique must be used throughout the procedure and at its termination in order to reduce the possibility of infection. The solution bag should remain in the carton and the overwrap intact ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 1.5% Dextrose 3000 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 1.5% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 2.5% Dextrose 3000 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 2.5% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 4.25% Dextrose 3000 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 4.25% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 1.5% Dextrose 2000 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 1.5% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 1.5% Dextrose 2500 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 1.5% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 2.5% Dextrose 2000 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 2.5% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 2.5% Dextrose 2500 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 2.5% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 4.25% Dextrose 2000 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 4.25% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – 4.25% Dextrose 2500 mL Bag Label - DELFLEX® PERITONEAL DIALYSIS SOLUTION - With - 4.25% DEXTROSE - LOW MAGNESIUM / LOW CALCIUM - and attached - stay•safe®Exchange Set ...
-
INGREDIENTS AND APPEARANCEProduct Information