Label: DELFLEX- dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solution
-
NDC Code(s):
46163-206-82,
46163-206-94,
46163-206-95,
46163-209-92, view more46163-209-94, 46163-209-95, 46163-212-92, 46163-212-94, 46163-212-95
- Packager: Fresenius Medical Care de Mexico, S.A. de C.V.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DELFLEX ®safely and effectively. See full prescribing information for DELFLEX ®peritoneal dialysis solution with attached stay•safe exchange set
DELFLEX (dextrose) peritoneal dialysis solution with attached stay•safe exchange set
Initial U.S. Approval: 1992INDICATIONS AND USAGE
For treatment of chronic kidney failure. ( 1)
DOSAGE AND ADMINISTRATION
For intraperitoneal dialysis only. ( 2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Adverse reactions may include peritonitis, catheter site infection, electrolyte and fluid imbalances, hypovolemia, hypervolemia, hypertension, disequilibrium syndrome, muscle cramping, abdominal pain, abdominal distension, and abdominal discomfort. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Medical Care North America at 1-800-323-5188 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Basic Dosing Information
2.2 Administration Instructions
2.3 Compatible Medications
3. Dosage Forms and Strengths
4. Contraindications
5. Warnings and Precautions
5.1 Electrolyte, Fluid and Nutrition Imbalances
5.2 Peritonitis and Encapsulating Peritoneal Sclerosis
5.2 Lactic Acidosis
5.4 Over Infusion
6. ADVERSE REACTIONS
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
2.1 Basic Dosing Information
DELFLEX® is intended for intraperitoneal administration only. Not for intravenous or intra-arterial administration.
The mode of therapy, frequency of treatment, formulation, exchange volume, duration of dwell, and length of dialysis should be selected by the physician responsible for the treatment of the individual patient.
Utilize the peritoneal dialysis solution with lowest level of osmolarity consistent with the fluid removal requirements for that exchange.
Do not store solutions containing additives.
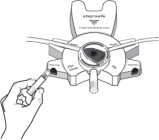
2.2 Administration Instructions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Do not heat in a microwave oven.
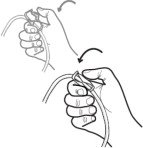
Get Ready
-
3. Dosage Forms and Strengths
DELFLEX peritoneal dialysis solutions are available in transparent single-dose flexible bags. The solution is clear with color being slightly yellow to colorless. All DELFLEX peritoneal dialysis solutions have overfills declared on the bag label. The flexible bag has the capacity for drainage in excess of their stated fill volume for ultrafiltration from the patient.
DELFLEX peritoneal dialysis solutions with an attached stay•safe exchange set are available in the sizes and formulations shown in Table 1.
Table 1. DELFLEX peritoneal dialysis solution with attached stay•safe exchange set sizes and formulations 2L 2.5L 3L DELFLEX Low Magnesium,
Low Calcium with 1.5% DextroseX X X DELFLEX Low Magnesium,
Low Calcium with 2.5% DextroseX X X DELFLEX Low Magnesium,
Low Calcium with 4.25% DextroseX X X - 4. Contraindications
-
5. Warnings and Precautions
5.1 Electrolyte, Fluid and Nutrition Imbalances
Peritoneal dialysis may affect a patient's protein, water-soluble vitamin, potassium, sodium, chloride, bicarbonate, and magnesium levels and volume status. Monitor electrolytes and blood chemistry periodically and take appropriate clinical action.
Potassium is omitted from DELFLEX solutions because dialysis may be performed to correct hyperkalemia. In situations where there is a normal serum potassium level or hypokalemia, the addition of potassium chloride (up to a concentration of 4 mEq/L) may be indicated to prevent severe hypokalemia.
To avoid the risk of severe dehydration or hypovolemia and to minimize the loss of protein, it is advisable to select the peritoneal dialysis solution with lowest level of osmolarity consistent with the fluid removal requirements for that exchange.
Significant loss of protein, amino acids and water-soluble vitamins may occur during peritoneal dialysis. Replacement therapy should be provided as necessary.
5.2 Peritonitis and Encapsulating Peritoneal Sclerosis
Infectious and aseptic peritonitis has been associated with peritoneal dialysis therapy. Following DELFLEX use, inspect the drained fluid for the presence of fibrin or cloudiness, which may indicate the presence of peritonitis. Improper clamping or priming sequence may result in infusion of air into the peritoneal cavity, which may result in abdominal pain and/or peritonitis. If peritonitis occurs, treat with appropriate therapy.
Encapsulating peritoneal sclerosis (EPS), sometimes fatal, is a complication of peritoneal dialysis therapy.
5.2 Lactic Acidosis
Monitor patients with conditions known to increase the risk of lactic acidosis [e.g., severe hypotension or sepsis that can be associated with acute kidney failure, inborn errors of metabolism, treatment with drugs such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs)] for lactic acidosis before the start of treatment and during treatment with DELFLEX.
Solutions containing the lactate ion should be used with great care in patients with metabolic or respiratory alkalosis. Lactate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
- 6. ADVERSE REACTIONS
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
DELFLEX solutions consist of electrolytes, lactate, and bicarbonate at physiological levels, and glucose to facilitate ultrafiltration. While there are no adequate and well controlled studies in pregnant women, appropriate administration of DELFLEX with monitoring of fluid, electrolyte, acid-base and glucose balance, is not expected to cause fetal harm. Animal reproduction studies have not been conducted with DELFLEX.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
-
11. DESCRIPTION
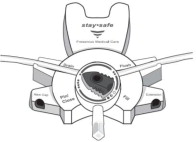
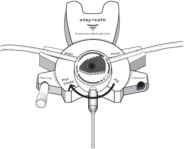
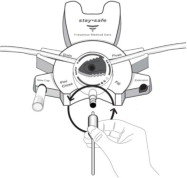
The DELFLEX® peritoneal dialysis solutions (low magnesium/low calcium) are sterile, non-pyrogenic formulations of dextrose and electrolytes in water for injection, USP, for use in peritoneal dialysis. These solutions do not contain antimicrobial agents or additional buffers. The stay•safe exchange set utilizes an easy to use dial designed to eliminate the use of clamps and to prevent touch contamination of internal connection components. Composition, calculated osmolarity, pH, and ionic concentrations are shown in Table 2.
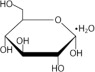
Table 2. Composition, calculated osmolarity, pH and ionic concentration Composition/100mL Total Osmolarity (mOsmoL/L) (calc) pH (5.0 - 6.0) Ionic Concentration (mEq/L) Dextrose Hydrous, USP (C 6H 12O 6•H 2O) Sodium Chloride, USP (NaCl) Sodium Lactate (C3H5NaO3) Calcium Chloride, USP (CaCl 2•2H 2O) Magnesium Chloride, USP (MgCl 2•6H 2O) Sodium Calcium Magnesium Chloride Lactate DELFLEX Low Magnesium, Low Calcium with 1.5% Dextrose 1.5 g 538 mg 448 mg 18.4 mg 5.08 mg 344 5.5 132 2.5 0.5 95 40 DELFLEX Low Magnesium, Low Calcium with 2.5% Dextrose 2.5 g 538 mg 448 mg 18.4 mg 5.08 mg 394 5.5 132 2.5 0.5 95 40 DELFLEX Low Magnesium, Low Calcium with 4.25% Dextrose 4.25g 538 mg 448 mg 18.4 mg 5.08 mg 483 5.5 132 2.5 0.5 95 40 Dextrose, USP, is chemically designated D-glucose monohydrate (C 6H 12O 6•H 2O) a hexose sugar freely soluble in water. The structural formula is shown here:
Calcium chloride, USP, is chemically designated calcium chloride dihydrate (CaCl 2•2H 2O) white fragments or granules freely soluble in water.
Magnesium chloride, USP, is chemically designated magnesium chloride hexahydrate (MgCl 2•6H 2O) colorless flakes or crystals very soluble in water.
Sodium lactate solution, USP, is chemically designated (CH 3CH(OH)COONa), a 60% aqueous solution miscible in water.
Sodium chloride, USP, is chemically designated (NaCl), a white, crystalline compound freely soluble in water.
Water for injection, USP, is chemically designated (H 2O).
Hydrochloric Acid or Sodium Hydroxide may be added for pH adjustment. pH is 5.5 ± 0.5.
Exposure to temperatures above 25°C (77°F) during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. Since the inner bag is compounded from a specific polyvinyl chloride, water may permeate from the inner bag into the overwrap in quantities insufficient to affect the solution significantly. Solutions in contact with the plastic inner bag can cause certain chemical components of the bag to leach out in very small amounts; however, the safety of the plastic formulation is supported by biological tests for plastic containers.
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
DELFLEX peritoneal dialysis solutions are hypertonic peritoneal dialysis solutions containing dextrose, a monosaccharide, as the primary osmotic agent. An osmotic gradient must be created between the peritoneal membrane and the dialysis solution in order for ultrafiltration to occur. The hypertonic concentration of glucose in DELFLEX solutions exert an osmotic pressure across the peritoneal membrane resulting in transcapillary ultrafiltration. Like other peritoneal dialysis solutions, DELFLEX solutions contain electrolytes to facilitate the correction of acid-base and electrolyte abnormalities. DELFLEX solutions contain a buffer, lactate, to help normalize acid-base abnormalities.
12.3 Pharmacokinetics
Absorption
Glucose can be rapidly absorbed from the peritoneal cavity by diffusion and appears quickly in the circulation due to the high glucose concentration gradient between DELFLEX solutions compared to blood capillary glucose level. Absorption per unit time will be the highest at the start of an exchange and decreases over time. The rate of glucose absorption will be dependent upon the transport characteristics of the patient's peritoneal membrane as determined by a peritoneal equilibration test (PET). Glucose absorption will also depend upon the concentration of glucose used for the exchange and the length of the dwell. Transport of other molecules will be dependent upon the molecular size of the solute, the concentration gradient, and the effective peritoneal surface area as determined by the PET.
Metabolism and Elimination
Glucose is metabolized by normal cellular pathways (i.e., glycolysis). Metabolism of lactate occurs in the liver and results in the generation of the bicarbonate. Glucose not absorbed during PD exchange procedure is removed by drainage of the PD solution from the peritoneal cavity.
- 13. NONCLINICAL TOXICOLOGY
-
16. HOW SUPPLIED/STORAGE AND HANDLING
DELFLEX peritoneal dialysis solutions with attached stay•safe exchange set are available in the sizes and formulations shown in Table 1[Dosage Forms and Strengths]. (3) DELFLEX peritoneal dialysis solutions are delivered in transparent, single-dose flexible bags. The solution is clear with color being slightly yellow to colorless.
Table 3. DELFLEX peritoneal dialysis with attached stay•safe exchange set NDC designations Magnesium (Mg); Calcium (Ca)
Unit Volume 2L 2.5L 3L Number of units per carton 5 5 4 Low Mg/Low Ca 1.5% Dextrose 46163-206-92 46163-206-94 46163-206-95 Low Mg/Low Ca 2.5% Dextrose 46163-209-92 46163-209-94 46163-209-95 Low Mg/Low Ca 4.25% Dextrose 46163-212-92 46163-212-94 46163-212-95 Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F). See USP Controlled Room Temperature. Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
Keep DELFLEX and all medicines out of the reach of children.
-
17. PATIENT COUNSELING INFORMATION
Aseptic technique must be used throughout the procedure and at its termination in order to reduce the possibility of infection.
The solution bag should remain in the carton and the overwrap intact until time of use.
Use only after checking for strength, clarity, amount, leaks, and expiration date.
Advise patients that DELFLEX peritoneal dialysis solution should not be heated in a microwave oven.
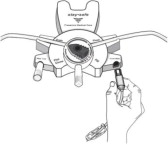
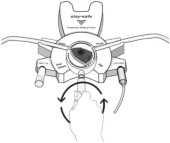
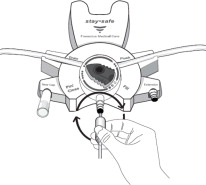
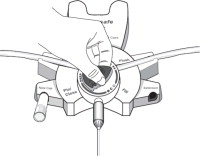
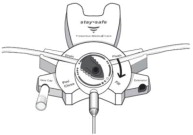
Disconnect from stay•safe DISC only when the blue dial is in position 4(●●●●) to ensure the fluid path of the stay•safe catheter extension set is sealed.
Care should be taken to ensure that there is not any leakage around the catheter, since if not controlled, the leakage can create edema from subcutaneous infiltration of the dialysis solution. The leakage will also create an inaccurate fluid balance measurement. If any leakage is identified, do not proceed with infusion and notify your physician.
Fresenius Medical Care North America
920 Winter Street
Waltham, MA 02451
1-800-323-5188
Made in Mexico© 2020 Fresenius Medical Care. All Rights Reserved. Fresenius Medical Care, triangle logo, stay•safe, DELFLEX, are trademarks of Fresenius Medical Care Holding, Inc. or its affiliated companies.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DELFLEX
dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:46163-206 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46163-206-82 5 in 1 CARTON 08/29/2024 1 2000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:46163-206-94 5 in 1 CARTON 08/29/2024 2 2500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:46163-206-95 4 in 1 CARTON 08/29/2024 3 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020171 08/29/2024 DELFLEX
dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:46163-209 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46163-209-92 5 in 1 CARTON 08/29/2024 1 2000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:46163-209-94 5 in 1 CARTON 08/29/2024 2 2500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:46163-209-95 4 in 1 CARTON 08/29/2024 3 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020171 08/29/2024 DELFLEX
dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:46163-212 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46163-212-95 5 in 1 CARTON 08/29/2024 1 2000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:46163-212-92 5 in 1 CARTON 08/29/2024 2 2000 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:46163-212-94 5 in 1 CARTON 08/29/2024 3 2500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020171 08/29/2024 Labeler - Fresenius Medical Care de Mexico, S.A. de C.V. (812652287) Registrant - Fresenius Medical Care North America (958291411)