Label: WALGREENS SENNA LAXATIVE- sennosides liquid
- NDC Code(s): 0363-7120-08
- Packager: WALGREENS CO.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
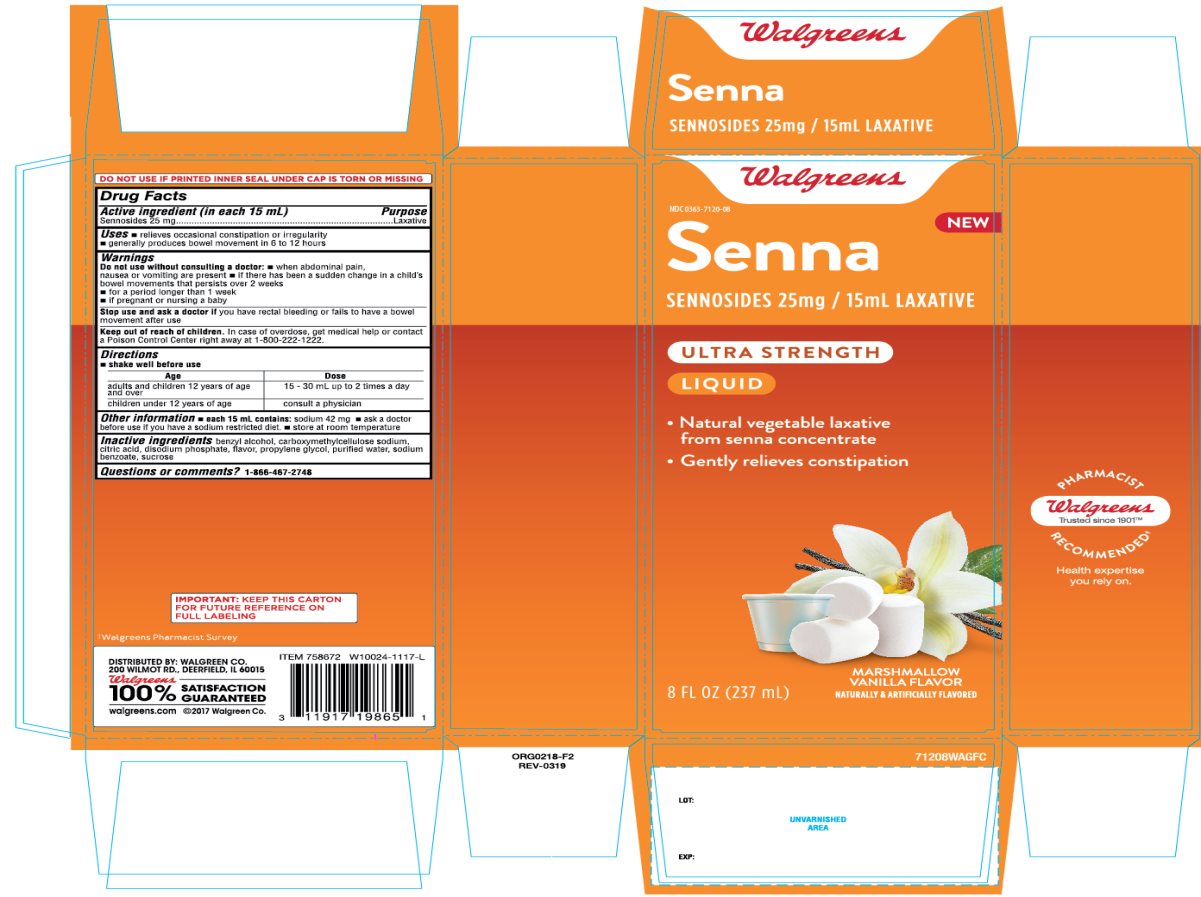
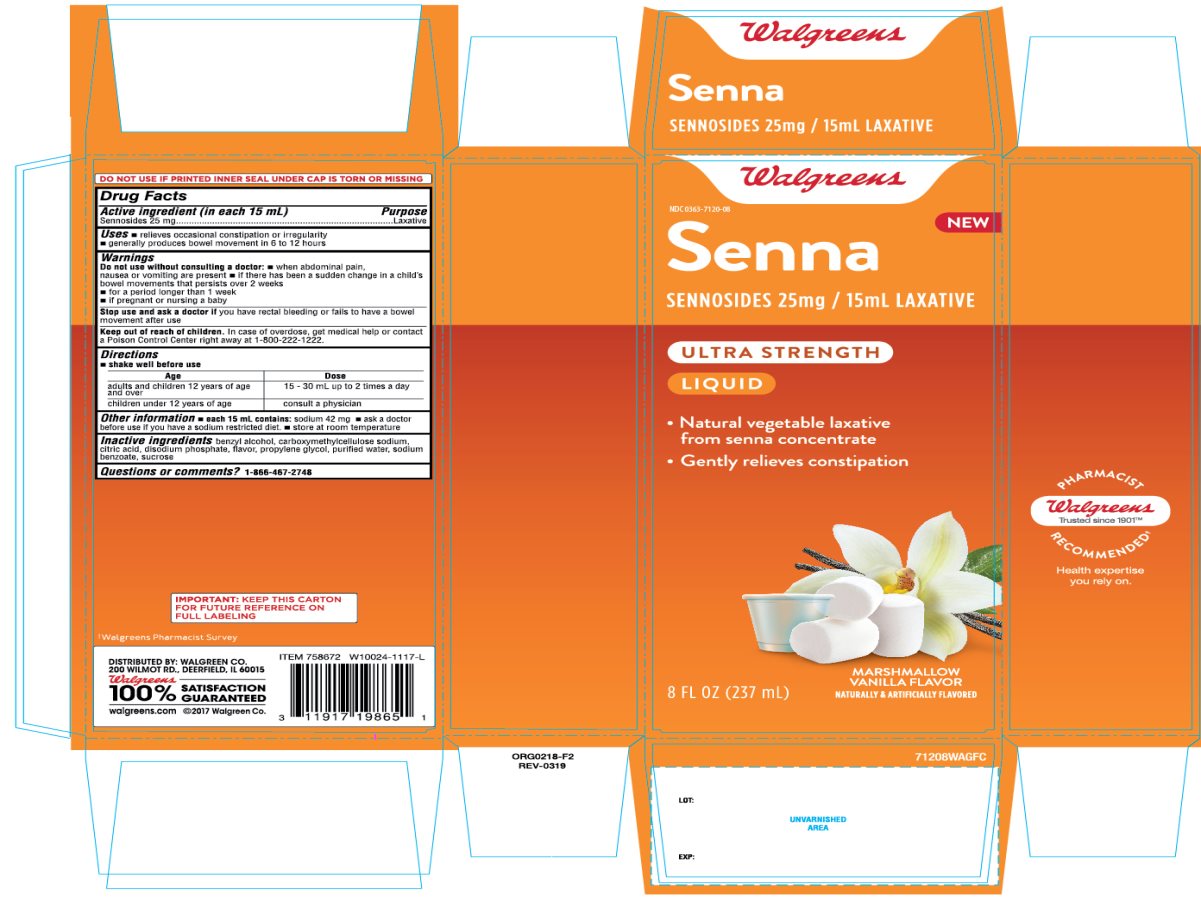
Principal Display Panel Senna Laxative
Walgreens
NDC 0363-7120-08
Senna
SENNOSIDES, 25 mg/ 15mL Laxative
ULTRA STRENGTH
LIQUID
- •
- Natural vegetable laxative from senna concentrate
- •
- Gently relieves constipation
Package Contains One Bottle
MARSHMALLOW VANILLA FLAVOR
NATURALLY AND ARTIFICIALLY FLAVOR
8 FL OZ (237 mL)
†Walgreens Pharmacist Survey
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
Walgreens
100% SATISFACTION GUARANTEED
walgreens.com © 2017 Walgreen Co.
IMPORTANT: Keep the carton for future reference on full labeling.
-
INGREDIENTS AND APPEARANCE
WALGREENS SENNA LAXATIVE
sennosides liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 25 mg in 15 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) Product Characteristics Color BROWN Score Shape Size Flavor MARSHMALLOW (VANILLA) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7120-08 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/09/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 04/09/2019 Labeler - WALGREENS CO. (008965063)