Label: NITROGLYCERIN tablet
- NDC Code(s): 70710-1018-1, 70710-1019-1, 70710-1019-4, 70710-1020-1
- Packager: Zydus Pharmaceuticals (USA) Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Nitroglycerin sublingual tablets, USP are stabilized sublingual compressed nitroglycerin tablets that contains 0.3 mg, 0.4 mg, or 0.6 mg nitroglycerin; as well as calcium stearate, colloidal silicon dioxide, glyceryl monostearate, lactose monohydrate and pregelatinized starch (botanical source: maize).
Nitroglycerin, USP is white crystalline powder.
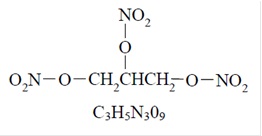
Nitroglycerin, an organic nitrate, is a vasodilating agent. The chemical name for nitroglycerin is 1, 2, 3 propanetriol trinitrate and the chemical structure is:
-
CLINICAL PHARMACOLOGY
The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of both arterial and venous beds. Dilation of postcapillary vessels, including large veins, promotes peripheral pooling of blood, decreases venous return to the heart, and reduces left ventricular end-diastolic pressure (preload). Nitroglycerin also produces arteriolar relaxation, thereby reducing peripheral vascular resistance and arterial pressure (afterload), and dilates large epicardial coronary arteries; however, the extent to which this latter effect contributes to the relief of exertional angina is unclear.
Therapeutic doses of nitroglycerin may reduce systolic, diastolic, and mean arterial blood pressure. Effective coronary perfusion pressure is usually maintained, but can be compromised if blood pressure falls excessively, or increased heart rate decreases diastolic filling time.
Elevated central venous and pulmonary capillary wedge pressures, and pulmonary and systemic vascular resistance are also reduced by nitroglycerin therapy. Heart rate is usually slightly increased, presumably due to a compensatory response to the fall in blood pressure. Cardiac index may be increased, decreased, or unchanged. Myocardial oxygen consumption or demand (as measured by the pressure-rate product, tension-time index, and stroke-work index) is decreased and a more favorable supply-demand ratio can be achieved. Patients with elevated left ventricular filling pressures and increased systemic vascular resistance in association with a depressed cardiac index are likely to experience an improvement in cardiac index. In contrast, when filling pressures and cardiac index are normal, cardiac index may be slightly reduced following nitroglycerin administration.
Mechanism of Action
Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3'5' monophosphate (cyclic GMP) in smooth muscle and other tissues. These events lead to dephosphorylation of myosin light chains, which regulate the contractile state in smooth muscle, and result in vasodilatation.
Pharmacodynamics
Consistent with the symptomatic relief of angina, digital plethysmography indicates that onset of the vasodilatory effect occurs approximately 1 to 3 minutes after sublingual nitroglycerin administration and reaches a maximum by 5 minutes postdose. Effects persist for at least 25 minutes following nitroglycerin sublingual tablets administration.
Pharmacokinetics and Drug Metabolism Absorption
Nitroglycerin is rapidly absorbed following sublingual administration of nitroglycerin sublingual tablets. Mean peak nitroglycerin plasma concentrations occur at a mean time of approximately 6 to 7 minutes postdose (Table 1). Maximum plasma nitroglycerin concentrations (Cmax) and area under the plasma concentration-time curves (AUC) increase dose-proportionally following 0.3 to 0.6 mg nitroglycerin sublingual tablets. The absolute bioavailability of nitroglycerin from nitroglycerin sublingual tablets is approximately 40% but tends to be variable due to factors influencing drug absorption, such as sublingual hydration and mucosal metabolism.
Table 1 Parameter
Mean Nitroglycerin (SD) Values
2 × 0.3 mg Nitroglycerin Sublingual Tablets
1 × 0.6 mg Nitroglycerin Sublingual Tablets
Cmax, ng/mL
2.3 (1.7)
2.1 (1.5)
Tmax, min
6.4 (2.5)
7.2 (3.2)
AUC(0–∞), min
14.9 (8.2)
14.9 (11.4)
t½, min
2.8 (1.1)
2.6 (0.6)
Distribution
The volume of distribution (VArea) of nitroglycerin following intravenous administration is 3.3 L/kg. At plasma concentrations between 50 and 500 ng/mL, the binding of nitroglycerin to plasma proteins is approximately 60%, while that of 1,2-and 1,3-dinitroglycerin is 60% and 30%, respectively.
Metabolism
A liver reductase enzyme is of primary importance in the metabolism of nitroglycerin to glycerol di-and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, 2 major metabolites 1,2-and 1,3-dinitroglycerin, are found in plasma. Mean peak 1,2-and 1,3-dinitroglycerin plasma concentrations occur at approximately 15 minutes postdose. The elimination half-life of 1,2-and 1,3-dinitroglycerin is 36 and 32 minutes, respectively. The 1,2-and 1,3-dinitroglycerin metabolites have been reported to possess approximately 2% and 10%, respectively, of the pharmacological activity of nitroglycerin. Higher plasma concentrations of the dinitro metabolites, along with their nearly 10-fold longer elimination half-lives, may contribute significantly to the duration of pharmacologic effect. Glycerol mononitrate metabolites of nitroglycerin are biologically inactive.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Nitroglycerin is contraindicated in patients who are allergic to it.
Sublingual nitroglycerin therapy is contraindicated in patients with early myocardial infarction, severe anemia, increased intracranial pressure, and those with a known hypersensitivity to nitroglycerin.
Administration of nitroglycerin sublingual tablets are contraindicated in patients who are using a phosphodiesterase-5 (PDE-5) inhibitor (e.g., sildenafil citrate, tadalafil, vardenafil hydrochloride) since these compounds have been shown to potentiate the hypotensive effects of organic nitrates.
Do not use nitroglycerin sublingual tablets in patients who are taking the soluble guanylate cyclase stimulator riociguat. Concomitant use can cause hypotension.
-
WARNINGS
The benefits of sublingual nitroglycerin in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use nitroglycerin in these conditions, careful clinical or hemodynamic monitoring must be used because of the possibility of hypotension and tachycardia.
-
PRECAUTIONS
Only the smallest dose required for effective relief of the acute anginal attack should be used. Excessive use may lead to the development of tolerance. Nitroglycerin sublingual tablets are intended for sublingual or buccal administration and should not be swallowed.
Severe hypotension, particularly with upright posture, may occur with small doses of nitroglycerin. This drug should therefore be used with caution in patients who may be volume-depleted or who, for whatever reason, are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris.
Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
As tolerance to other forms of nitroglycerin develops, the effects of sublingual nitroglycerin on exercise tolerance, although still observable, is blunted.
In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance rarely occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence.
Several clinical trials of nitroglycerin patches or infusions in patients with angina pectoris have evaluated regimens that incorporated a 10- to 12- hour nitrate free interval. In some of these trials, an increase in the frequency of anginal attacks during the nitrate free interval was observed in a small number of patients. In one trial, patients had decreased exercise tolerance at the end of the nitrate interval. Hemodynamic rebound has been observed only rarely; on the other hand, few studies were so designed that rebound, if it had occurred, would have been detected.
Nitrate tolerance as a result of sublingual nitroglycerin administration is probably possible, but only in patients who maintain high continuous nitrate levels for more than 10 or 12hours daily. Such use of sublingual nitroglycerin would entail administration of scores of tablets daily and is not recommended.
The drug should be discontinued if blurring of vision or drying of the mouth occurs. Excessive dosage of nitroglycerin may produce severe headaches.
Information for Patients
Nitroglycerin sublingual tablets should not be chewed, crushed, or swallowed.
If possible, patients should sit down when taking nitroglycerin sublingual tablets and should use caution when returning to a standing position. This eliminates the possibility of falling due to lightheadedness or dizziness.
One tablet should be dissolved under the tongue or in the buccal pouch at the first sign of an acute anginal attack. The dose may be repeated approximately every 5 minutes until relief is obtained.
If chest pain persists after a total of 3 tablets in a 15 minute period, or if the pain is different than is typically experienced, prompt medical attention is recommended.
Nitroglycerin sublingual tablets may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack.
Nitroglycerin may produce a burning or tingling sensation when administered sublingually; however, the ability to produce a burning or tingling sensation should not be considered a reliable method for determining the potency of the tablets.
Headaches can sometimes accompany treatment with nitroglycerin. In patients who get these headaches, the headaches may be a marker of the activity of the drug.
Treatment with nitroglycerin may be associated with lightheadedness upon standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
Nitroglycerin sublingual tablets should be kept in the original glass container and must be tightly capped after each use to prevent loss of tablet potency.
Drug Interactions
Concomitant use of nitroglycerin sublingual tablets with soluble guanylate cyclase stimulators is contraindicated (see CONTRAINDICATIONS).
Concomitant use of nitrates and alcohol may cause hypotension.
The vasodilatory and hemodynamic effects of nitroglycerin may be enhanced by concomitant administration of aspirin.
Intravenous administration of nitroglycerin decreases the thrombolytic effect of alteplase. Therefore, caution should be observed in patients receiving sublingual nitroglycerin during alteplase therapy.
Intravenous nitroglycerin reduces the anticoagulant effect of heparin and activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single sublingual nitroglycerin doses.
Tricyclic antidepressants (amitriptyline, desipramine, doxepin, others) and anticholinergic drugs may cause dry mouth and diminished salivary secretions. This may make dissolution of sublingual nitroglycerin difficult. Increasing salivation with chewing gum or artificial saliva products may prove useful in aiding dissolution of sublingual nitroglycerin.
Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
Administration of nitroglycerin is contraindicated in patients who are using PDE-5 inhibitors (e.g., sildenafil citrate, tadalafil, vardenafil hydrochloride). These compounds have been shown to potentiate the hypotensive effects of organic nitrates.
A decrease in therapeutic effect of sublingual nitroglycerin may result from use of long-acting nitrates.
Drug/Laboratory Test Interactions
Nitrates may interfere with the Zlatkis-Zak color reaction, causing a false report of decreased serum cholesterol.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed.
Carcinogenicity potential of nitroglycerin was evaluated in rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years. Rats developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At high dose, the incidences of hepatocellular carcinomas in males was 48% and in females was 33%, compared to 0% in untreated controls. Incidences of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
Nitroglycerin was mutagenic in Ames tests performed in 2 different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, PO, or in ex vivo cytogenetic tests in rat and dog cells.
In a 3-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for 6 months prior to mating of the F0 generation, with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this 3-generation study, there was no clear evidence of teratogenicity.
Pregnancy Category B
Animal reproduction and teratogenicity studies have not been conducted with nitroglycerin sublingual tablets. However, teratology studies conducted in rats and rabbits with topically applied nitroglycerin ointment at dosages up to 80 mg/kg/day and 240 mg/kg/day, respectively revealed no toxic effects on dams or fetuses.
There are no adequate and well-controlled studies in pregnant women. Nitroglycerin should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nitroglycerin is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of nitroglycerin in pediatric patients have not been established.
Geriatric Use
Clinical studies of nitroglycerin sublingual tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Headache that may be severe and persistent may occur immediately after use. Vertigo, dizziness, weakness, palpitation, and other manifestations of postural hypotension may develop occasionally, particularly in erect, immobile patients. Marked sensitivity to the hypotensive effects of nitrates (manifested by nausea, vomiting, weakness, diaphoresis, pallor, and collapse) may occur at therapeutic doses. Syncope due to nitrate vasodilatation has been reported. Flushing, drug rash, and exfoliative dermatitis have been reported in patients receiving nitrate therapy.
-
OVERDOSAGE
Hemodynamic Effects
The effects of nitroglycerin overdose are generally the results of nitroglycerin's capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; tachycardia; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures; and death.
No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia
Methemoglobinemia has been rarely reported in association with organic nitrates. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial PO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
If methemoglobinemia is present, intravenous administration of methylene blue, 1 to 2 mg/kg of body weight, may be required.
-
DOSAGE AND ADMINISTRATION
One tablet should be dissolved under the tongue or in the buccal pouch at the first sign of an acute anginal attack. The dose may be repeated approximately every 5 minutes until relief is obtained. If the pain persists after a total of 3 tablets in a 15-minute period, or if the pain is different than is typically experienced, prompt medical attention is recommended. Nitroglycerin sublingual tablets, USP may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack.
During administration the patient should rest, preferably in the sitting position.
No dosage adjustment is required in patients with renal failure.
-
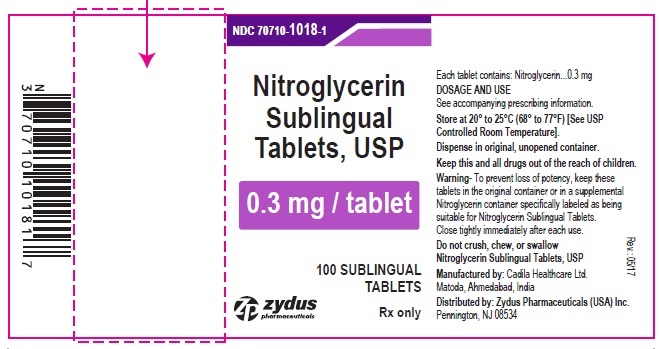
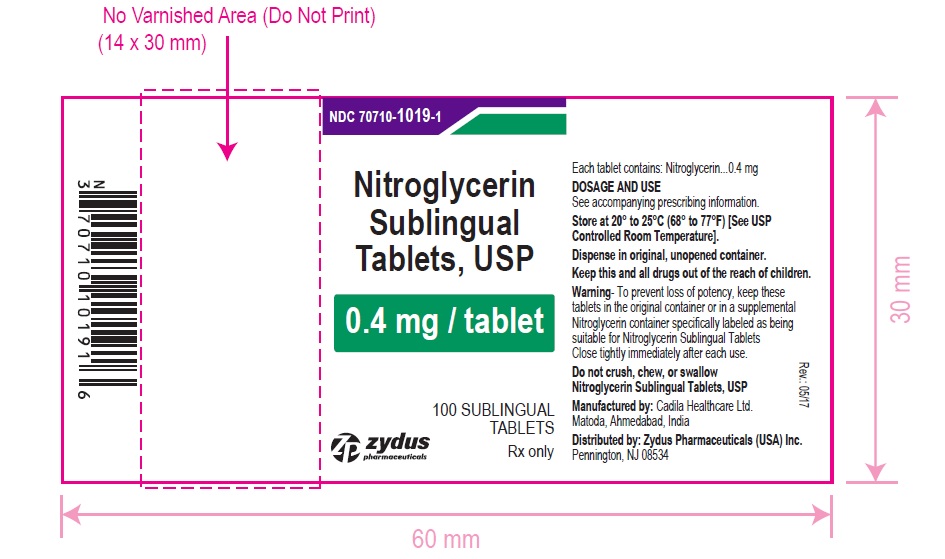
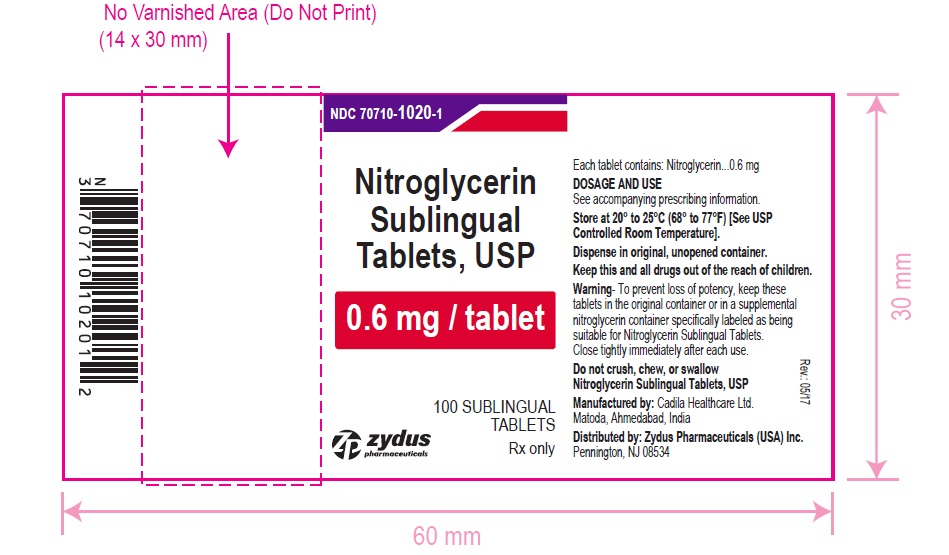
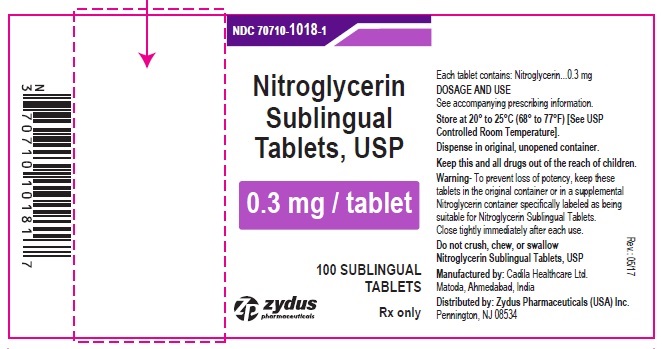
HOW SUPPLIED
Nitroglycerin Sublingual Tablets USP, 0.3 mg are white to off-white, round, flat face, uncoated tablets, debossed with "3" on one side & "T" on other side and are supplied as:
NDC 68382-1018-1 in bottle of 100 sublingual tablets
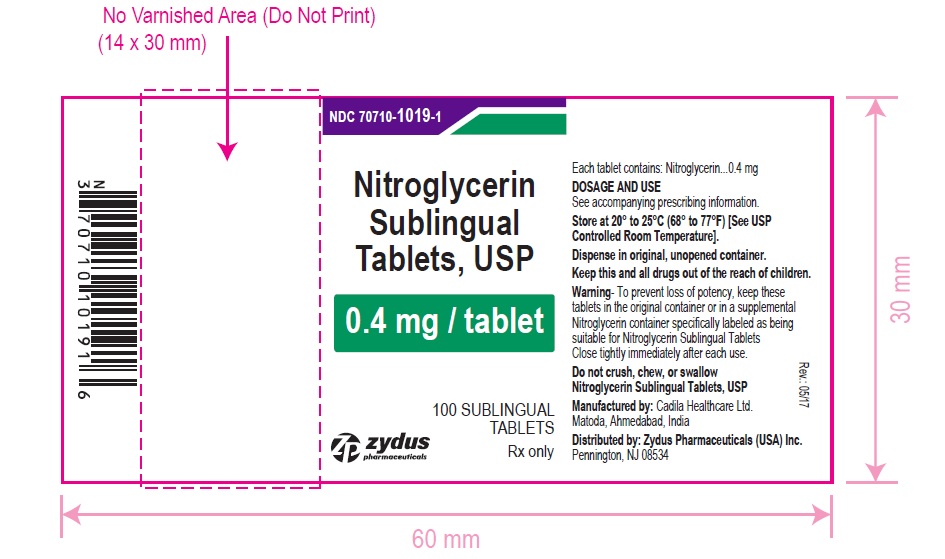
Nitroglycerin Sublingual Tablets USP, 0.4 mg are white to off-white, round, flat face, uncoated tablets, debossed with "4" on one side and "T" on other side and are supplied as:
NDC 70710-1019-1 in bottle of 100 sublingual tablets
NDC 70710-1019-4 in patient convenience package of 100 tablets (4 bottles of 25 sublingual tablets)
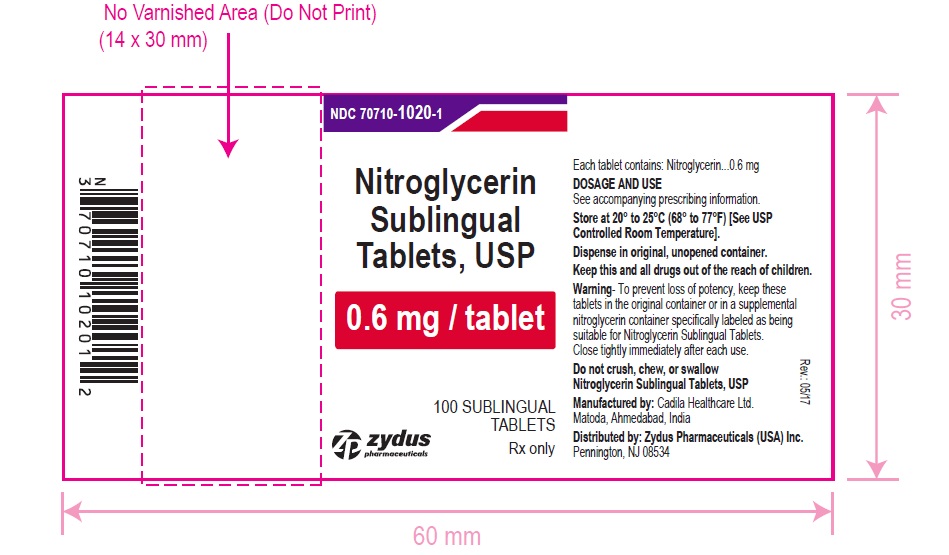
Nitroglycerin Sublingual Tablets USP, 0.6 mg are white to off-white, round, flat face, uncoated tablets, debossed with "6" on one side and "T" on other side and are supplied as:
NDC 70710-1020-1 in bottle of 100 sublingual tablets
Store between 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature].
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Nitroglycerin (nī′trō-glis′ĕr-in) Sublingual Tablets, USP
Read this information carefully before you start nitroglycerin (nī′trō-glis′ĕr-in) sublingual tablets and each time you refill your prescription. There may be new information. This information does not replace talking with your doctor. If you have any questions about nitroglycerin sublingual tablets, ask your doctor. Your doctor will know if nitroglycerin sublingual tablets are right for you.
What are nitroglycerin sublingual tablets?
Nitroglycerin sublingual tablets are a type of medicine known as an organic nitrate and are a vasodilating agent. It is used to treat a type of chest pain called angina.
What is Angina?
Angina is a pain or discomfort that keeps coming back when part of your heart does not get enough blood. Angina feels like a pressing or squeezing pain, usually in your chest under the breastbone. Sometimes you can feel it in your shoulders, arms, neck, jaws, or back. Nitroglycerin sublingual tablets can relieve this pain.
Who should not use nitroglycerin sublingual tablets?
Do not use nitroglycerin sublingual tablets if you are allergic to organic nitrates (like the active ingredient in nitroglycerin sublingual tablets).
You should not take nitroglycerin sublingual tablets if you have the following conditions:
- very recent heart attack
- severe anemia
- increased pressure in the head
Do not take nitroglycerin sublingual tablets with drugs for erectile dysfunction, like VIAGRA® (sildenafil citrate), CIALIS® (tadalafil), or LEVITRA® (vardenafil hydrochloride), as this may lead to extreme lowering of your blood pressure.
Do not take nitroglycerin sublingual tablets if you take medicines called guanylate cyclase stimulators which include riociguat, a medicine that treats pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension.
What should I tell my doctor before taking nitroglycerin sublingual tablets?
Before using nitroglycerin sublingual tablets, tell your doctor if:
- You are taking any medicines that are used to treat angina, heart failure, or an irregular heartbeat.
- You are taking any medicines that reduce blood pressure.
- You are taking any diuretics (water pills).
- You are taking medications to treat depression or psychiatric illness.
- You are taking ergotamine or similar drugs for migraine headaches.
- You are taking aspirin.
- You are taking the blood thinner medicine heparin.
- You are taking any medicines for erectile dysfunction.
- You are pregnant or plan to become pregnant.
- You are breastfeeding.
How should I take nitroglycerin sublingual tablets?
- Do not chew, crush, or swallow nitroglycerinsublingual tablets .
- You should sit down when taking nitroglycerinsublingual tablets and use caution when you stand up. This eliminates the possibility of falling due to lightheadedness or dizziness.
- One tablet should be dissolved under the tongue or in the oral cavity at the first sign of chest pain.
- The dose may be repeated approximately every 5 minutes, until the chest pain is relieved.
- If the pain persists after a total of 3 tablets in a 15 minute period, or is different than you typically experience, call your doctor or seek emergency help.
- Nitroglycerinsublingual tablets may be used 5 to 10 minutes prior to activities that might cause chest pain.
- You may feel a burning or tingling sensation in your mouth when you take nitroglycerinsublingual tablets .
What should I avoid while taking nitroglycerin sublingual tablets?
- Do not breastfeed. It is not known if nitroglycerinsublingual tablets will pass through your milk.
- Do not consume alcohol while taking nitroglycerinsublingual tablets , as this can lower your blood pressure.
- Do not start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first.
What are the possible side effects of nitroglycerin sublingual tablets?
Nitroglycerin sublingual tablets may cause the following side effects:
- headache
- vertigo (a major symptom of balance disorder)
- dizziness
- weakness
- heart palpitations (unusual awareness of the heartbeat)
- low blood pressure upon rising from a seated position
- nausea and vomiting
- sweating
- paleness
- fainting
- flushing (warm or red condition of your skin)
- other skin reactions that may be severe
Nitroglycerin sublingual tablets may cause a false test result of decreased serum cholesterol.
Tell your doctor if you are concerned about any side effects you experience. These are not all the possible side effects of nitroglycerin sublingual tablets. For a complete list, ask your doctor or pharmacist.
How do I store nitroglycerin sublingual tablets?
Nitroglycerin sublingual tablets should be kept in the original glass container and tightly capped after each use to prevent loss of tablet potency.
Store nitroglycerin sublingual tablets at room temperature (between 68° and 77°F).
General advice about nitroglycerin sublingual tablets
Sometimes doctors will prescribe a medicine for a condition that is not included in the patient information leaflets. Only use nitroglycerin sublingual tablets the way your doctor told you to. Do not give nitroglycerin sublingual tablets to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about nitroglycerin sublingual tablets.
Please address medical inquiries to, MedicalAffairs@zydususa.com Tel.: 1-877-993-8779.
Brand listed are registered trademark of their respective owners.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Patient Information has been approved by the U.S. Food and Drug Administration.
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NITROGLYCERIN
nitroglycerin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70710-1019 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 0.4 mg Inactive Ingredients Ingredient Name Strength CALCIUM STEARATE (UNII: 776XM7047L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (WHITE TO OFF WHITE) Score no score Shape ROUND (ROUND) Size 4mm Flavor Imprint Code 4;T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70710-1019-1 1 in 1 CARTON 02/02/2023 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:70710-1019-4 4 in 1 CARTON 02/02/2023 2 25 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210153 02/02/2023 NITROGLYCERIN
nitroglycerin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70710-1020 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 0.6 mg Inactive Ingredients Ingredient Name Strength CALCIUM STEARATE (UNII: 776XM7047L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (WHITE TO OFF WHITE) Score no score Shape ROUND (ROUND) Size 4mm Flavor Imprint Code 6;T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70710-1020-1 1 in 1 CARTON 02/02/2023 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210153 02/02/2023 NITROGLYCERIN
nitroglycerin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70710-1018 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 0.3 mg Inactive Ingredients Ingredient Name Strength CALCIUM STEARATE (UNII: 776XM7047L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (WHITE TO OFF WHITE) Score no score Shape ROUND (ROUND) Size 4mm Flavor Imprint Code 3;T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70710-1018-1 1 in 1 CARTON 02/02/2023 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210153 02/02/2023 Labeler - Zydus Pharmaceuticals (USA) Inc. (156861945) Registrant - Zydus Pharmaceuticals (USA) Inc. (156861945) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 863362789 ANALYSIS(70710-1018, 70710-1019, 70710-1020) , MANUFACTURE(70710-1018, 70710-1019, 70710-1020)