Label: ACETAZOLAMIDE injection, powder, lyophilized, for solution
- NDC Code(s): 39822-0190-1
- Packager: XGen Pharmaceuticals DJB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
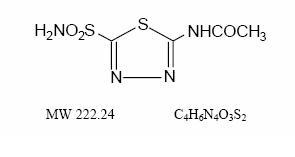
Acetazolamide, an inhibitor of the enzyme carbonic anhydrase, is a white to faintly yellowish white crystalline, odorless powder, weakly acidic, very slightly soluble in water and slightly soluble ...
-
CLINICAL PHARMACOLOGY
Acetazolamide is a potent carbonic anhydrase inhibitor, effective in the control of fluid secretion (e.g., some types of glaucoma), in the treatment of certain convulsive disorders (e.g. ...
-
INDICATIONS AND USAGE
For adjunctive treatment of: edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies (petit mal, unlocalized seizures); chronic simple (open-angle) glaucoma ...
-
CONTRAINDICATIONS
Hypersensitivity to acetazolamide or any excipients in the formulation. Since acetazolamide is a sulfonamide derivative, cross sensitivity between acetazolamide, sulfonamides and other sulfonamide ...
-
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis ...
-
PRECAUTIONS
General - Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under ...
-
ADVERSE REACTIONS
Body as a whole: Headache, malaise, fatigue, fever, pain at injection site, flushing, growth retardation in children, flaccid paralysis, anaphylaxis - Digestive: Gastrointestinal disturbances ...
-
OVERDOSAGE
No specific antidote is known. Treatment should be symptomatic and supportive. Electrolyte imbalance, development of an acidotic state, and central nervous effects might be expected to occur ...
-
DOSAGE AND ADMINISTRATION
Preparation and Storage of Parenteral Solution: Each 500 mg vial containing sterile acetazolamide sodium should be reconstituted with at least 5 mL of Sterile Water for Injection prior to use ...
-
HOW SUPPLIED
Intravenous Acetazolamide for Injection USP (lyophilized) powder. NDC 39822-0190-1, 500 mg Vial - Store drug product at controlled room temperature 20°- 25°C (68°- 77°F). Reconstituted solution ...
-
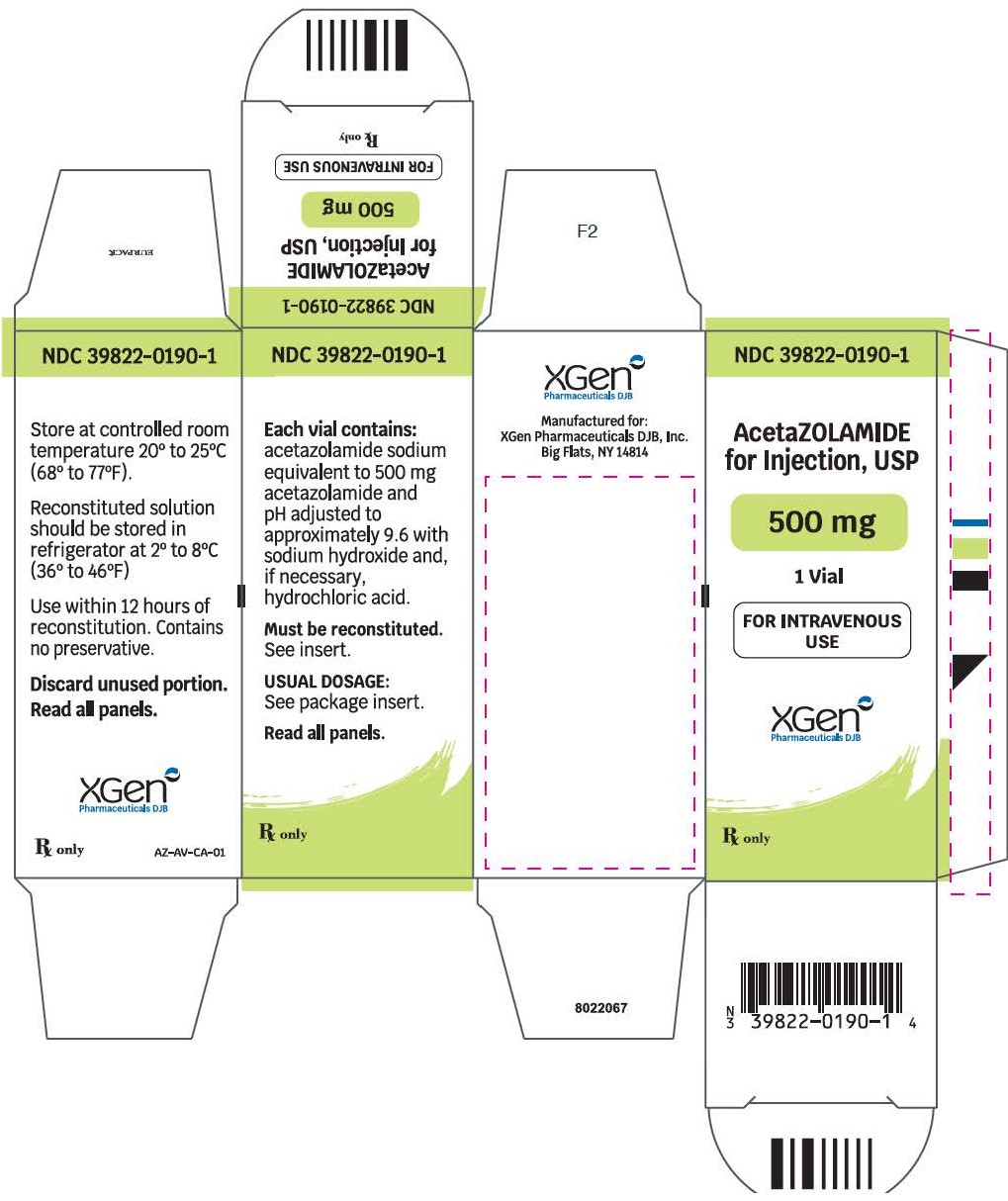
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC:39822-0190-1 - Acetazolamide for Injection, USP - 500 mg Acetazolamide Base Activity - For Intravenous Use - Rx Only - Single Vial Carton - XGen Pharmaceuticals DJB ...
-
INGREDIENTS AND APPEARANCEProduct Information