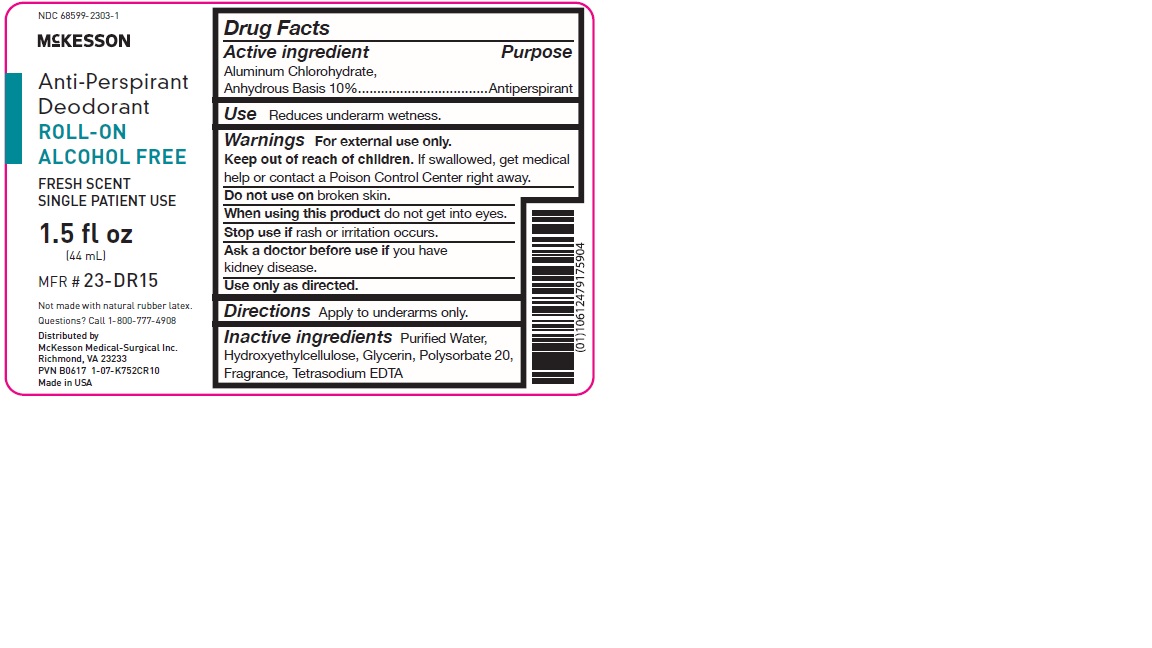
Label: ROLL ON ANTI-PERSPIRANT (ALCOHOL FREE)- aluminum chlorohydrate solution
- NDC Code(s): 68599-2303-1
- Packager: McKesson Medical-Surgical, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROLL ON ANTI-PERSPIRANT (ALCOHOL FREE)
aluminum chlorohydrate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-2303 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 20 (UNII: 7T1F30V5YH) EDETATE SODIUM (UNII: MP1J8420LU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-2303-1 45 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 10/19/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 08/05/2013 Labeler - McKesson Medical-Surgical, Inc. (023904428)