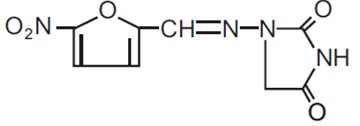Label: NITROFURANTOIN capsule
- NDC Code(s): 62332-389-31, 62332-389-91, 62332-390-31, 62332-390-91, view more
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of nitrofurantoin capsules, USP (macrocrystals)and other antibacterial drugs, nitrofurantoin capsules, USP ...
-
DESCRIPTIONNitrofurantoin macrocrystals, USP is a synthetic chemical of controlled crystal size. It is a stable, brown yellow, macrocrystalline compound. Nitrofurantoin macrocrystals, USP is an antibacterial ...
-
CLINICAL PHARMACOLOGYNitrofurantoin macrocrystals are a larger crystal form of nitrofurantoin. The absorption of nitrofurantoin macrocrystals is slower and its excretion somewhat less when compared to nitrofurantoin ...
-
INDICATIONS AND USAGENitrofurantoin capsules, USP (macrocrystals) is specifically indicated for the treatment of urinary tract infections when due to susceptible strains of Escherichia coli, enterococci ...
-
CONTRAINDICATIONSAnuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of ...
-
WARNINGSPulmonary reactions: ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN MACROCRYSTALS SHOULD BE ...
-
PRECAUTIONSInformation for Patients: Patients should be advised to take nitrofurantoin capsules (macrocrystals) with food to further enhance tolerance and improve drug absorption. Patients should be ...
-
ADVERSE REACTIONSRespiratory: CHRONIC, SUBACUTE, OR ACUTE PULMONARY HYPERSENSITIVITY REACTIONS MAY OCCUR. CHRONIC PULMONARY REACTIONS OCCUR GENERALLY IN PATIENTS WHO HAVE RECEIVED CONTINUOUS TREATMENT FOR SIX ...
-
OVERDOSAGEOccasional incidents of acute overdosage of nitrofurantoin capsules (macrocrystals) have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no ...
-
DOSAGE AND ADMINISTRATIONNitrofurantoin capsules (macrocrystals) should be given with food to improve drug absorption and, in some patients, tolerance. Adults: 50 to 100 mg four times a day - the lower dosage level is ...
-
HOW SUPPLIEDNitrofurantoin Capsules, USP (Macrocrystals), 25 mg are white opaque cap / white opaque body, size 4, hard gelatin capsules imprinted with “A219” on cap in black ink, filled with light yellow to ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 25 mgNDC 62332-389-31 - Nitrofurantoin Capsules, USP (Macrocrystals) 25 mg - URINARY TRACT ANTIBACTERIAL - Rx only - 100 Capsules - Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 50 mgNDC 62332-390-31 - Nitrofurantoin Capsules, USP (Macrocrystals) 50 mg - URINARY TRACT ANTIBACTERIAL - Rx only - 100 Capsules - Alembic
-
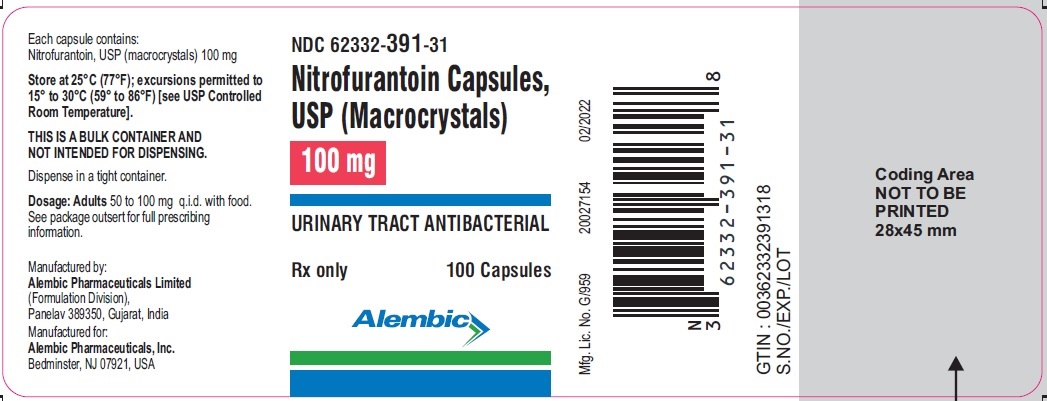
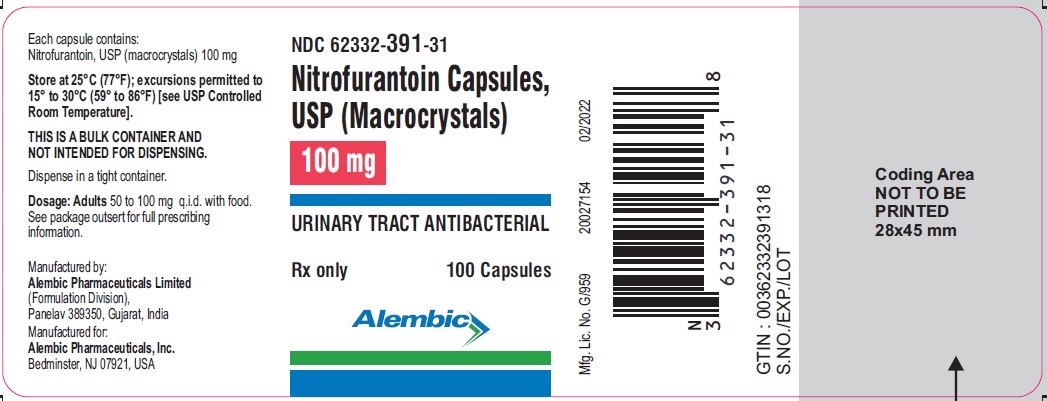
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 100 mgNDC 62332-391-31 - Nitrofurantoin Capsules, USP (Macrocrystals) 100 mg - URINARY TRACT ANTIBACTERIAL - Rx only - 100 Capsules - Alembic
-
INGREDIENTS AND APPEARANCEProduct Information