Label: EDARAVONE injection, solution
- NDC Code(s): 39822-4500-1, 39822-4500-2, 39822-4510-1
- Packager: XGen Pharmaceuticals DJB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EDARAVONE INJECTION safely and effectively. See full prescribing information for EDARAVONE INJECTION. EDARAVONE injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Edaravone injection is indicated for the treatment of amyotrophic lateral sclerosis (ALS).
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information - The recommended dosage of edaravone injection is as follows: an intravenous infusion of 60 mg administered over a 60-minute period. Administer edaravone injection ...
-
3 DOSAGE FORMS AND STRENGTHS
Edaravone injection is supplied for intravenous infusion in a single-dose USP Type 1 Glass Vial containing - 30 mg or - 60 mgof edaravone in 100 mL of clear, colorless aqueous solution.
-
4 CONTRAINDICATIONS
Edaravone injection is contraindicated in patients with a history of hypersensitivity to edaravone or any of the inactive ingredients in this product. Hypersensitivity reactions and anaphylactic ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions - Hypersensitivity reactions (redness, wheals, and erythema multiforme) and cases of anaphylaxis (urticaria, decreased blood pressure, and dyspnea) have been ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions - [see - WARNINGS AND PRECAUTIONS (5.1)] Sulfite Allergic Reactions - [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of edaravone injection in pregnant women. In animal studies, administration of ...
-
11 DESCRIPTION
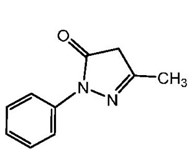
The active ingredient in edaravone injection is edaravone, which is a member of the substituted 2-pyrazolin-5-one class. The chemical name of edaravone is [3-methyl-1-phenyl-2-pyrazolin-5-one] ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism by which edaravone injection exerts its therapeutic effect in patients with ALS is unknown. 12.2 Pharmacodynamics - Cardiac Electrophysiology - At ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies of edaravone using the intravenous route have not been conducted. Mutagenesis - Edaravone ...
-
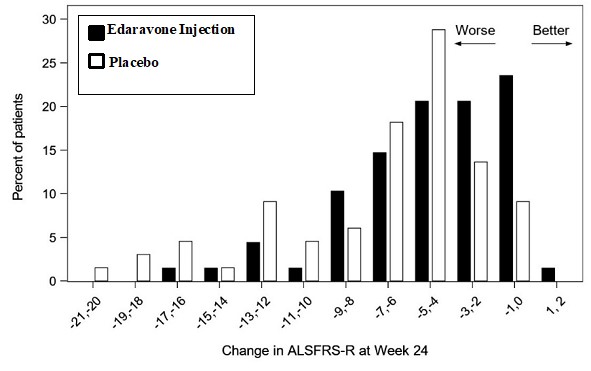
14 CLINICAL STUDIES
The efficacy of edaravone injection for the treatment of ALS was established in a 6-month, randomized, placebo-controlled, double-blind study conducted in Japanese patients with ALS who were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
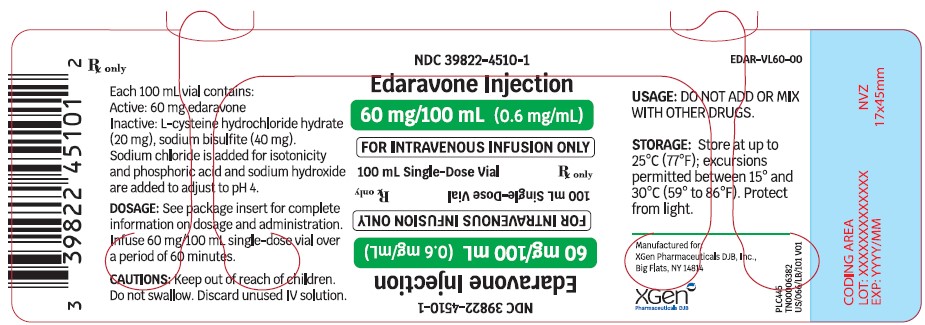
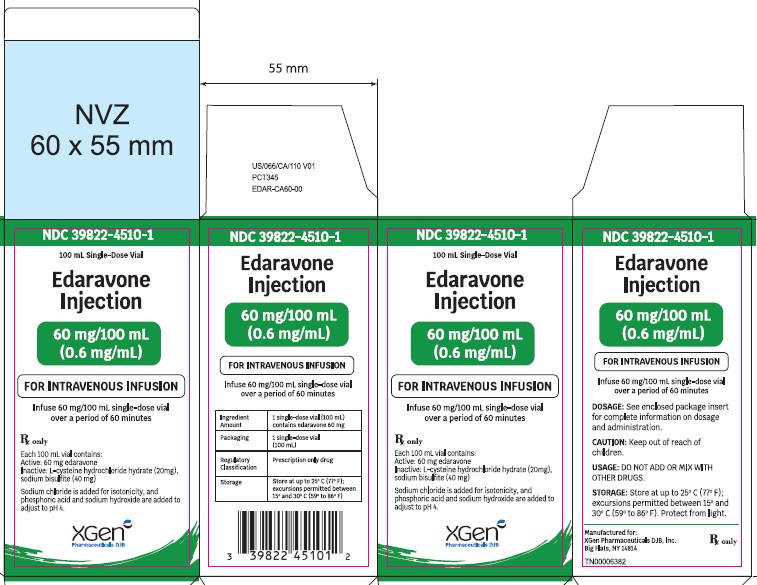
16.1 How Supplied - Edaravone injection is supplied as a 30 mg/100 mL (0.3 mg/mL) clear, colorless, sterile solution for intravenous infusion in single-dose USP Type 1 Glass Vial - [see ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patients to read the FDA-approved patient labeling (Patient Information). Hypersensitivity Reactions - Advise patients to seek immediate medical care if they experience signs or ...
-
PATIENT INFORMATION
EDARAVONE INJECTION - (e-dar-a-vone) For Intravenous Infusion - What is edaravone injection? Edaravone injection is a prescription medicine used to treat people with amyotrophic lateral sclerosis ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information