Label: CETIRIZINE HYDROCHLORIDE tablet, chewable
- NDC Code(s): 0781-5284-06, 0781-5284-64
- Packager: Sandoz Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Do not use if you have ever had an allergic reactions to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
- •
- if breast-feeding: not recommended
- •
- if pregnant: ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
- •
- may be taken with or without water
- •
- Chew or crush tablets completely before swallowing
adults and children 6 years and over
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
-
Inactive ingredients
acesulfame potassium, artificial flavors, benzyl alcohol, betadex, colloidal silicon dioxide, dl-alpha-tocopherol, ferric oxide red, ferric oxide yellow, lactose monohydrate, magnesium stearate, maltodextrin, microcrystalline cellulose, propylene glycol, talc and tutti frutti flavor
Questions? 1-800-525-8747
Product of India
Manufactured by Sandoz Private Ltd.,
for Sandoz Inc., Princeton, NJ 08540
Rev.10/2024
-
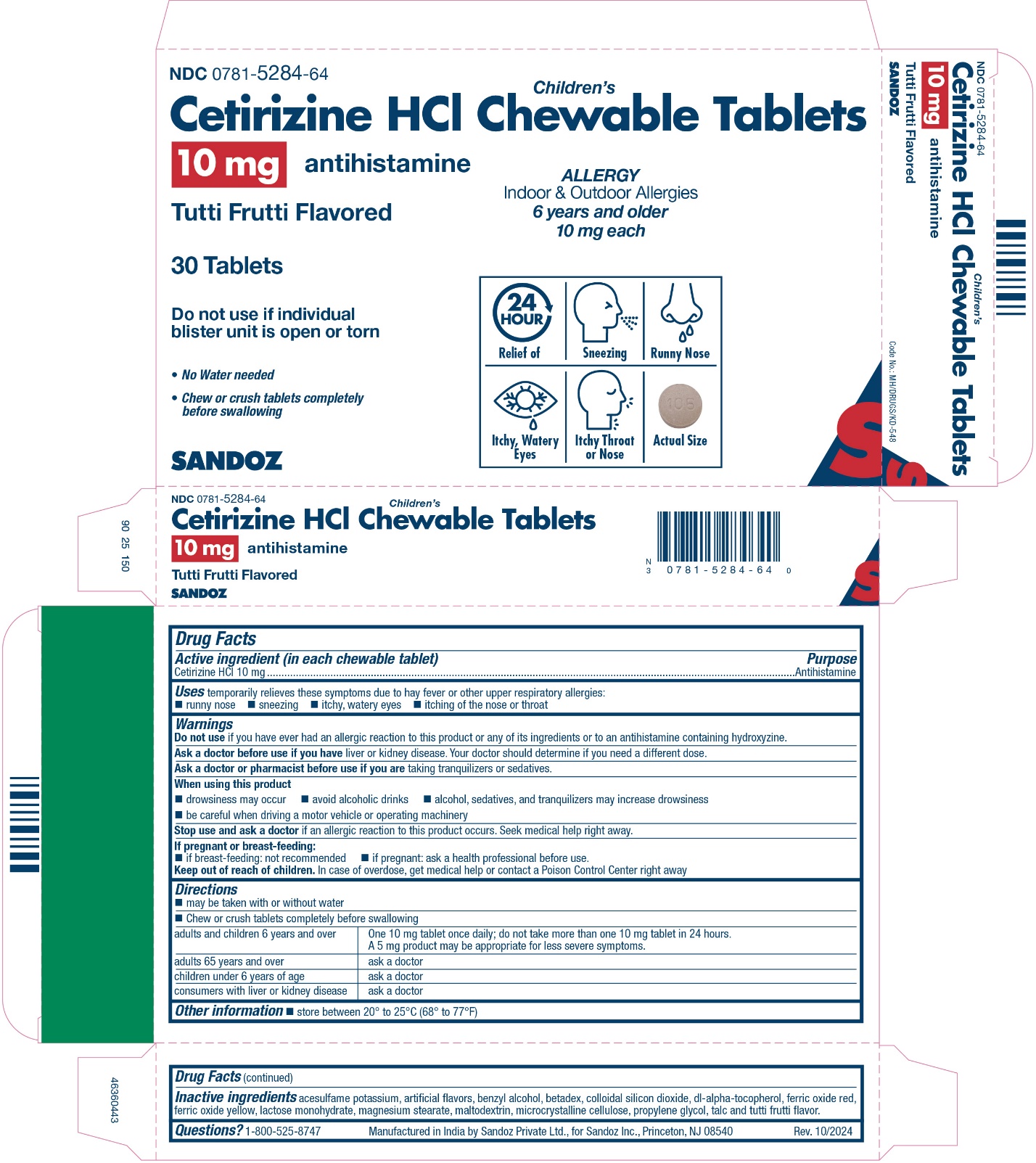
Principal Display Panel
NDC 0781-5284-64
Children's
Cetirizine HCl Chewable Tablets
10 mg
antihistamine
Tutti Frutti Flavored
30 Tablets
Do not use if individual blister unit is open or torn
ALLERGY
Indoor & Outdoor Allergies
24 hour Relief of
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy, Watery Eyes
- •
- Itchy Throat or Nose
- No Water needed
- Chew or crush tablets completely before swallowing
6 yrs. and older 10 mg each
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0781-5284 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) BENZYL ALCOHOL (UNII: LKG8494WBH) BETADEX (UNII: JV039JZZ3A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color YELLOW (peach, mottled) Score no score Shape ROUND Size 9mm Flavor Imprint Code SZ;106 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0781-5284-64 30 in 1 CARTON 02/14/2008 1 NDC:0781-5284-06 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078692 02/14/2008 Labeler - Sandoz Inc (005387188)