Label: ERGOCALCIFEROL capsule, liquid filled
- NDC Code(s): 70518-0177-0, 70518-0177-1, 70518-0177-2, 70518-0177-3, view more
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 69452-151
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
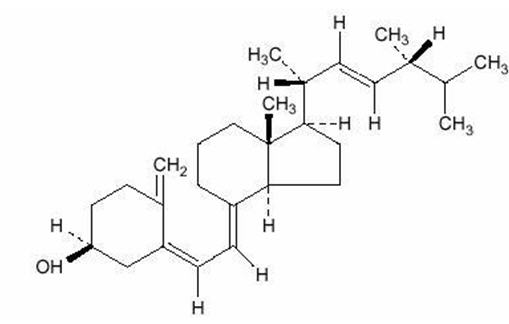
DESCRIPTIONERGOCALCIFEROL CAPSULES, USP, is a synthetic calcium regulator for oral administration. Ergocalciferol is a white, colorless crystal, insoluble in water, soluble in organic solvents, and slightly ...
-
CLINICAL PHARMACOLOGYThe - in vivo synthesis of the major biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of ergocalciferol takes place in the liver (to 25-hydroxyvitamin ...
-
INDICATIONS AND USAGEErgocalciferol is indicated for use in the treatment of hypoparathyroidism, refractory rickets, also known as vitamin D resistant rickets, and familial hypophosphatemia.
-
CONTRAINDICATIONSErgocalciferol is contraindicated in patients with hypercalcemia, malabsorption syndrome, abnormal sensitivity to the toxic effects of vitamin D, and hypervitaminosis D.
-
WARNINGSHypersensitivity to vitamin D may be one etiologic factor in infants with idiopathic hypercalcemia. In these cases vitamin D must be strictly restricted. Keep out of the reach of children.
-
PRECAUTIONSGeneral - Vitamin D administration from fortified foods, dietary supplements, self-administered and prescription drug sources should be evaluated. Therapeutic dosage should be readjusted as soon ...
-
ADVERSE REACTIONSHypervitaminosis D is characterized by effects on the following organ system: Renal:Impairment of renal function with polyuria, nocturia, polydipsia, hypercalciuria, reversible azotemia ...
-
OVERDOSAGEThe effects of administered vitamin D can persist for two or more months after cessation of treatment. Hypervitaminosis D is characterized by: Hypercalcemia with anorexia, nausea, weakness ...
-
DOSAGE AND ADMINISTRATIONTHE RANGE BETWEEN THERAPEUTIC AND TOXIC DOSES IS NARROW. Vitamin D Resistant Rickets:12,000 to 500,000 USP units daily. Hypoparathyroidism:50,000 to 200,000 USP units daily concomitantly with ...
-
HOW SUPPLIEDEach green, oval softgel is imprinted with PA140 and contains 1,250 mcg (50,000 USP units vitamin D) of ergocalciferol, USP, and is available in - NDC: 70518-0177-00 - NDC: 70518-0177-01 - NDC ...
-
PRINCIPAL DISPLAY PANELDRUG: Ergocalciferol - GENERIC: Ergocalciferol - DOSAGE: CAPSULE, LIQUID FILLED - ADMINSTRATION: ORAL - NDC: 70518-0177-0 - NDC: 70518-0177-1 - NDC: 70518-0177-2 - NDC: 70518-0177-3 - NDC: 70518-0177-4 - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information