Label: ASPIRIN LOW DOSE- aspirin tablet, delayed release
- NDC Code(s): 55910-563-17
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
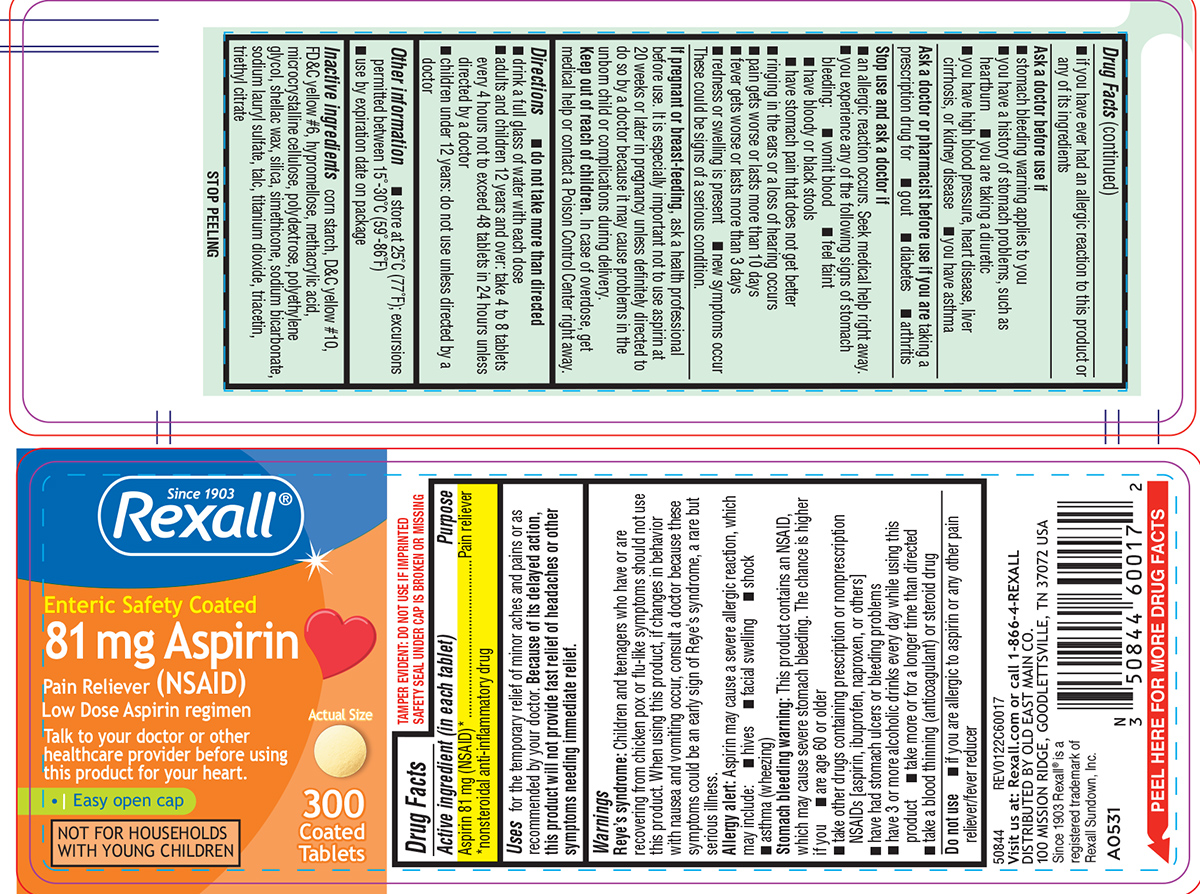
Active ingredient (in each tablet)
Aspirin 81 mg (NSAID)* *nonsteroidal anti-inflammatory drug
-
Purpose
Pain reliever
-
Uses
for the temporary relief of minor aches and pains or as recommended by your doctor. Because of its delayed action, this product will not provide fast relief of headaches or other symptoms needing ...
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea ...
-
Directions
do not take more than directed - drink a full glass of water with each dose - adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours ...
-
Other information
store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) use by expiration date on package
-
Inactive ingredients
corn starch, D&C yellow #10, FD&C yellow #6, hypromellose, methacrylic acid, microcrystalline cellulose, polydextrose, polyethylene glycol, shellac wax, silica, simethicone, sodium bicarbonate ...
-
Principal display panel
Since 1903 - Rexall® Enteric Safety Coated - 81 mg Aspirin - Pain Reliever (NSAID) Low Dose Aspirin regimen - Talk to your doctor or other - healthcare provider before using - this product for your ...
-
INGREDIENTS AND APPEARANCEProduct Information