Label: OSCEOLA SUPPLY INC 6176- chloroxylenol soap
- NDC Code(s): 62672-300-01, 62672-300-03, 62672-300-05, 62672-300-07, view more
- Packager: OSCEOLA SUPPLY, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
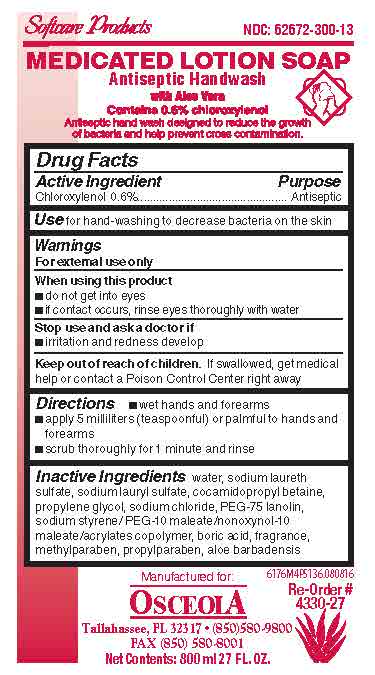
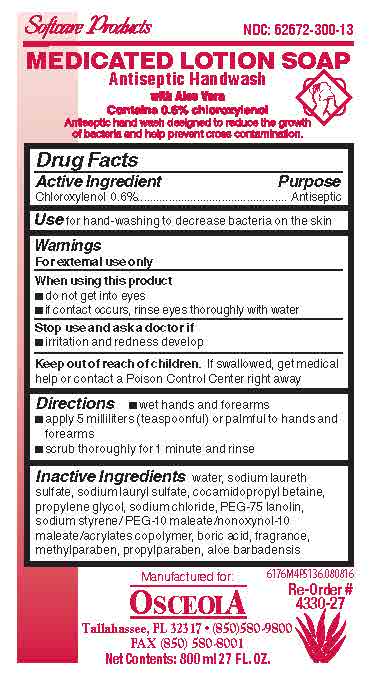
Drug Facts Box OTC-Active Ingredient SectionChloroxylenol 0.6%
-
Drug Facts Box OTC-Purpose SectionAntiseptic
-
Drug Facts Box OTC-Indications & Usage Sectionfor hand-washing to decrease bacteria on the skin
-
Drug Facts Box OTC-Warnings SectionFor external use only
-
Drug Facts Box OTC-When Using Sectiondo not get into eyes - if contact occurs, rinse eyes thoroughly with water
-
Drug Facts Box OTC-Stop Use Sectionirritation and redness develop
-
Drug Facts Box OTC-Keep Out of Reach of Children Sectionif swallowed, get medical help or contact a Poison Control Center right away
-
Drug Facts Box OTC-Dosage & Administration Sectionwet hands and forearms - apply 5 milliliters (teaspoonful) or palmful to hands and forearms - scrub thoroughly for 1 minute and rinse
-
Drug Facts Box OTC-Inactive Ingredient Sectionwater, sodium laureth sulfate, sodium lauryl sulfate, cocamidopropyl betaine, propylene glycol, sodium chloride, PEG-75 lanolin, sodium styrene/PEG-10 maleate/nonoxynol-10 maleate/acrylates ...
-
Medicated Lotion Soap 6176 LabelMedicated Lotion Soap 6176 - 6176M4P5136.jpg
-
INGREDIENTS AND APPEARANCEProduct Information

 6176M4P5136.jpg
6176M4P5136.jpg