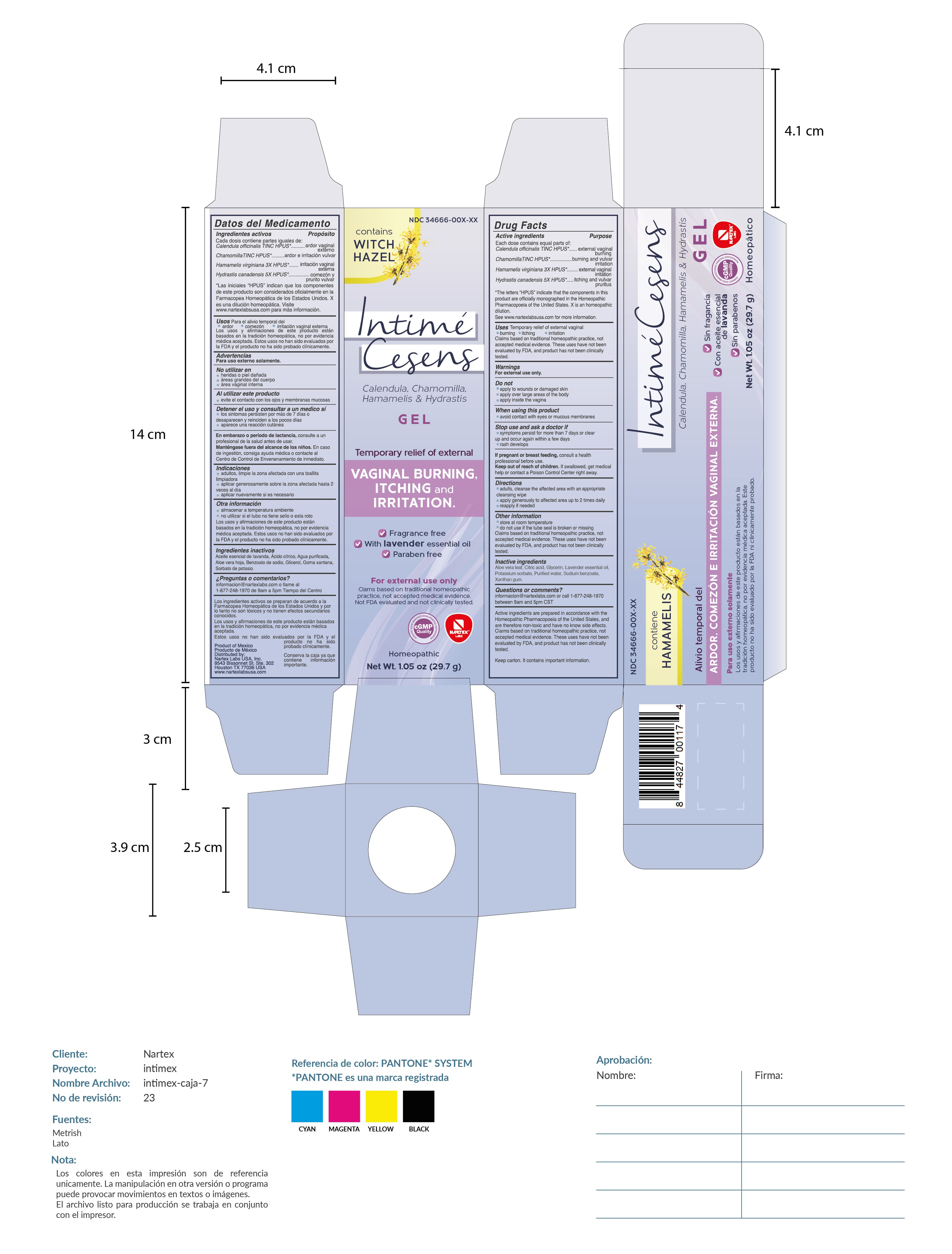
Label: INTIME CESENS gel
- NDC Code(s): 34666-301-01
- Packager: Nartex Laboratorios Homeopaticos SA DE CV
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INTIME CESENS
- Drug Facts
- Active Ingredient
- Purpose
- USES
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- KEEP OUT OF REACH OF CHILDREN
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
Active Ingredients are prepared in accordance with the Homeopathic Pharmacopoeia of the United States, and are therefore non-toxic and have no known side effects. Claims based on traditional homeopathic practice, not accepted medical evidence. These uses have not been evaluated by FDA, and product has not been clnically tested.
Keep carton. It contains important information.
Product of Mexico / Producto de Mexico
Distributed by: Nartex Labs, USA, Inc.
9543 Bissonnet St. STE. 302
Houston, TX 77036 USA
www.nartexlabsusa.com
-
PRINCIPAL DISPLAY PANEL
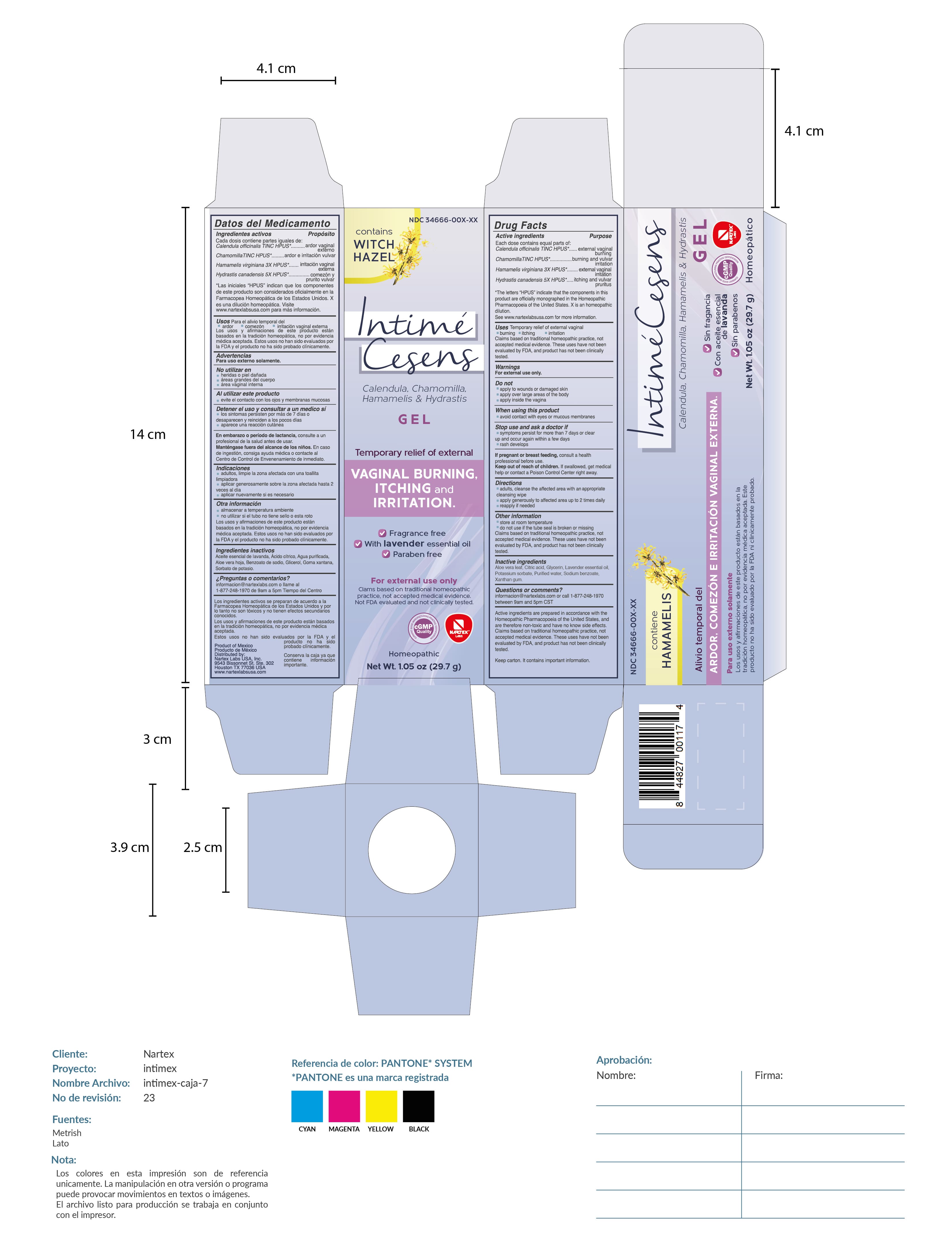 Contains witch hazel
Contains witch hazel
Intime Cesens
Calendula, Chamomila, Hamamelis & Hydrastis Gel
Temporary relief of external vaginal burning, itching and irritation.
fragrance free. with lavender essential oil. paraben free
For external use only
Claims based on traditional homeopathic practice, not acceptd medical evidence. Not FDA evaluated and not clinically tested.
Homeopathic
Net Wt. 1.05oz (29.7g)
-
INGREDIENTS AND APPEARANCE
INTIME CESENS
intime cesens gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:34666-301 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDRASTIS CANADENSIS WHOLE (UNII: R763EBH88T) (HYDRASTIS CANADENSIS WHOLE - UNII:R763EBH88T) HYDRASTIS CANADENSIS WHOLE 5 [hp_X] in 1 g HAMAMELIS VIRGINIANA WHOLE (UNII: V663Q8TEFU) (HAMAMELIS VIRGINIANA WHOLE - UNII:V663Q8TEFU) HAMAMELIS VIRGINIANA WHOLE 3 [hp_X] in 1 g MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 1 [hp_X] in 1 g CALENDULA OFFICINALIS WHOLE (UNII: PFR03EBU0H) (CALENDULA OFFICINALIS WHOLE - UNII:PFR03EBU0H) CALENDULA OFFICINALIS WHOLE 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength LAVENDER OIL (UNII: ZBP1YXW0H8) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color Score Shape FREEFORM Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:34666-301-01 29.7 g in 1 TUBE; Type 0: Not a Combination Product 06/11/2020 11/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/11/2020 11/19/2024 Labeler - Nartex Laboratorios Homeopaticos SA DE CV (589914576) Registrant - Nartex Laboratorios Homeopaticos SA DE CV (589914576) Establishment Name Address ID/FEI Business Operations Nartex Laboratorios Homeopaticos SA DE CV 589914576 manufacture(34666-301)