Label: CROMOLYN SODIUM solution, concentrate
- NDC Code(s): 81665-104-96
- Packager: Omnivium Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR ORAL USE ONLY-NOT FOR INHALATION OR INJECTION. MUST BE DILUTED.
-
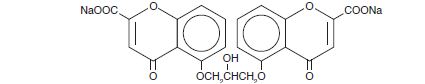
DESCRIPTION:Each 5 mL ampule of Cromolyn Sodium Oral Solution (Concentrate) contains 100 mg cromolyn sodium, USP, in purified water. Cromolyn sodium is a hygroscopic, white powder having little odor. It may ...
-
CLINICAL PHARMACOLOGY:In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the release of mediators from sensitized mast cells. Cromolyn sodium acts by inhibiting the release of histamine ...
-
CLINICAL STUDIES:Four randomized, controlled clinical trials were conducted with Cromolyn Sodium Oral Solution (Concentrate) in patients with either cutaneous or systemic mastocytosis; two of which utilized a ...
-
INDICATIONS AND USAGE:Cromolyn Sodium Oral Solution (Concentrate) is indicated in the management of patients with mastocytosis. Use of this product has been associated with improvement in diarrhea, flushing, headaches ...
-
CONTRAINDICATIONS:Cromolyn Sodium Oral Solution (Concentrate) is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium.
-
WARNINGS: The recommended dosage should be decreased in patients with decreased renal or hepatic function. Severe anaphylactic reactions may occur rarely in association with cromolyn sodium ...
-
PRECAUTIONS:In view of the biliary and renal routes of excretion of Cromolyn Sodium Oral Solution (Concentrate), consideration should be given to decreasing the dosage of the drug in patients with impaired ...
-
ADVERSE REACTIONS:Most of the adverse events reported in mastocytosis patients have been transient and could represent symptoms of the disease. The most frequently reported adverse events in mastocytosis patients ...
-
DOSAGE AND ADMINISTRATION:NOT FOR INHALATION OR INJECTION. SEE DIRECTIONS FOR USE. The usual starting dose is as follows: Adults and Adolescents (13 Years and Older): Two ampules four times daily, taken one-half hour ...
-
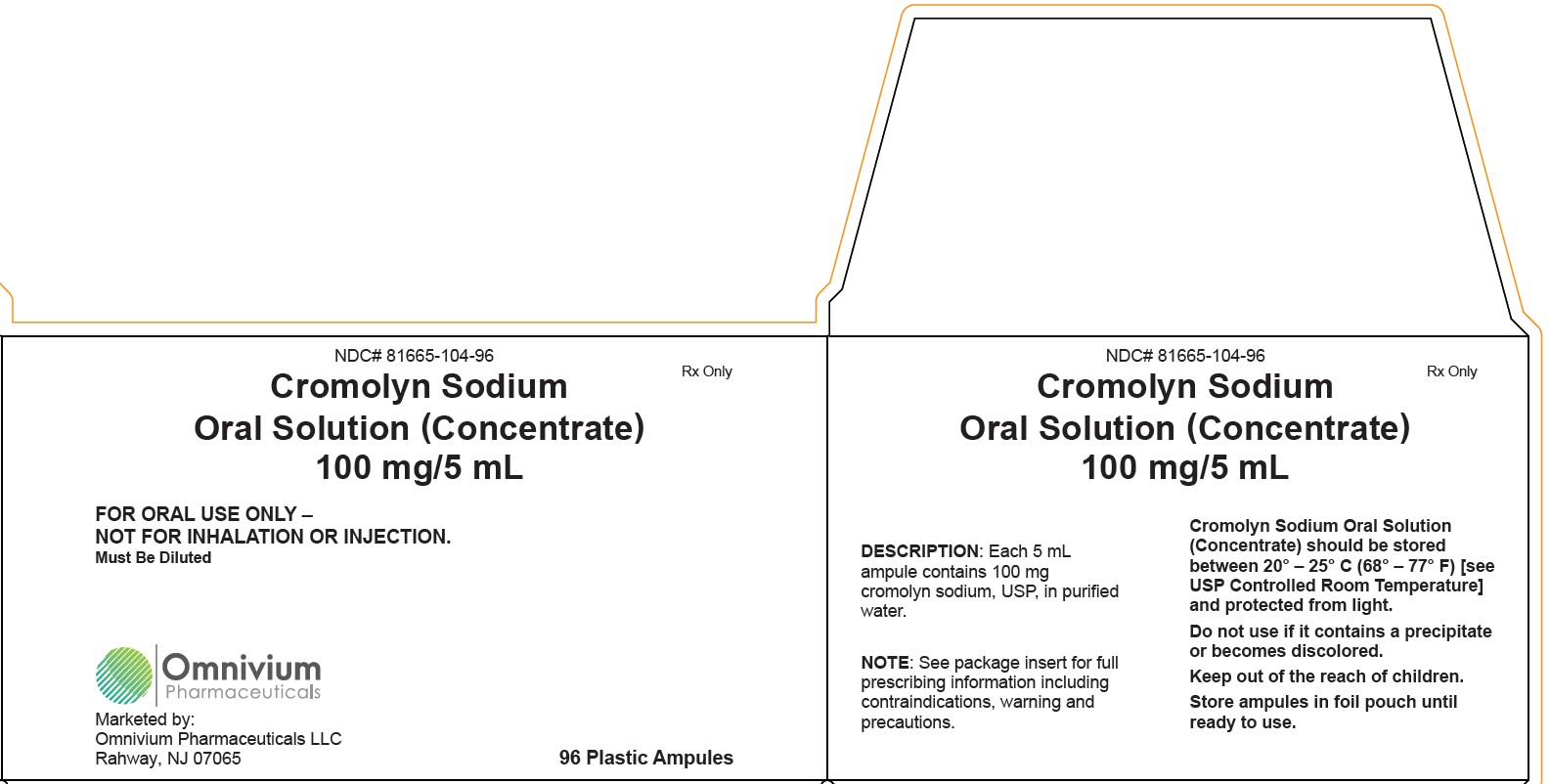
HOW SUPPLIED:Cromolyn Sodium Oral Solution (Concentrate) is an unpreserved, colorless solution supplied in a low density polyethylene plastic unit dose ampule with 8 ampules per foil pouch. Each 5 mL ampule ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Omnivium Pharmaceuticals LLC - Rahway, NJ 07065 - Rev.: May 2024 - RPIN0198
-
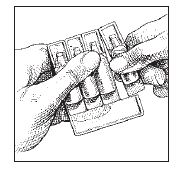
Patient InstructionsCromolyn Sodium - Oral Solution (Concentrate) FOR ORAL USE ONLY – NOT FOR INHALATION OR INJECTION. MUST BE DILUTED. How to Use Cromolyn Sodium Oral Solution (Concentrate): As with all ...
-
Cromolyn Sodium Oral Solution (Concentrate), 100 mg/5 mL Carton LabelNDC 81665-104-96 - Rx Only - Cromolyn Sodium - Oral Solution (Concentrate) 100 mg/5 mL - FOR ORAL USE ONLY – NOT ...
-
INGREDIENTS AND APPEARANCEProduct Information


 LDPE).
LDPE).