Label: ATROPINE SULFATE solution
- NDC Code(s): 70069-631-01, 70069-631-25, 70069-641-01, 70069-641-25
- Packager: Somerset Therapeutics, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ATROPINE SULFATE INJECTION safely and effectively. See full prescribing information for ATROPINE SULFATE INJECTION. ATROPINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAtropine Sulfate Injection, USP, is indicated for temporary blockade of severe or life threatening muscarinic effects, e.g., as an antisialagogue, an antivagal agent, an antidote for ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Administration - Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do ...
-
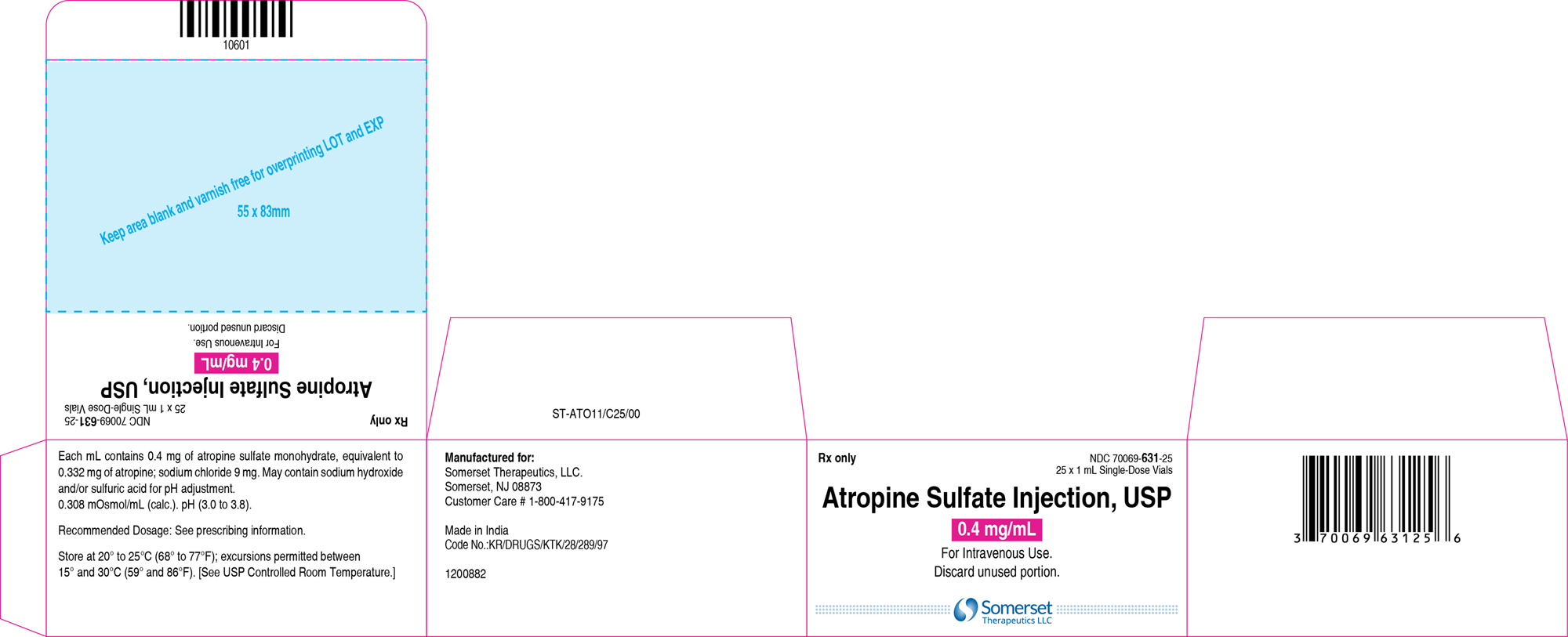
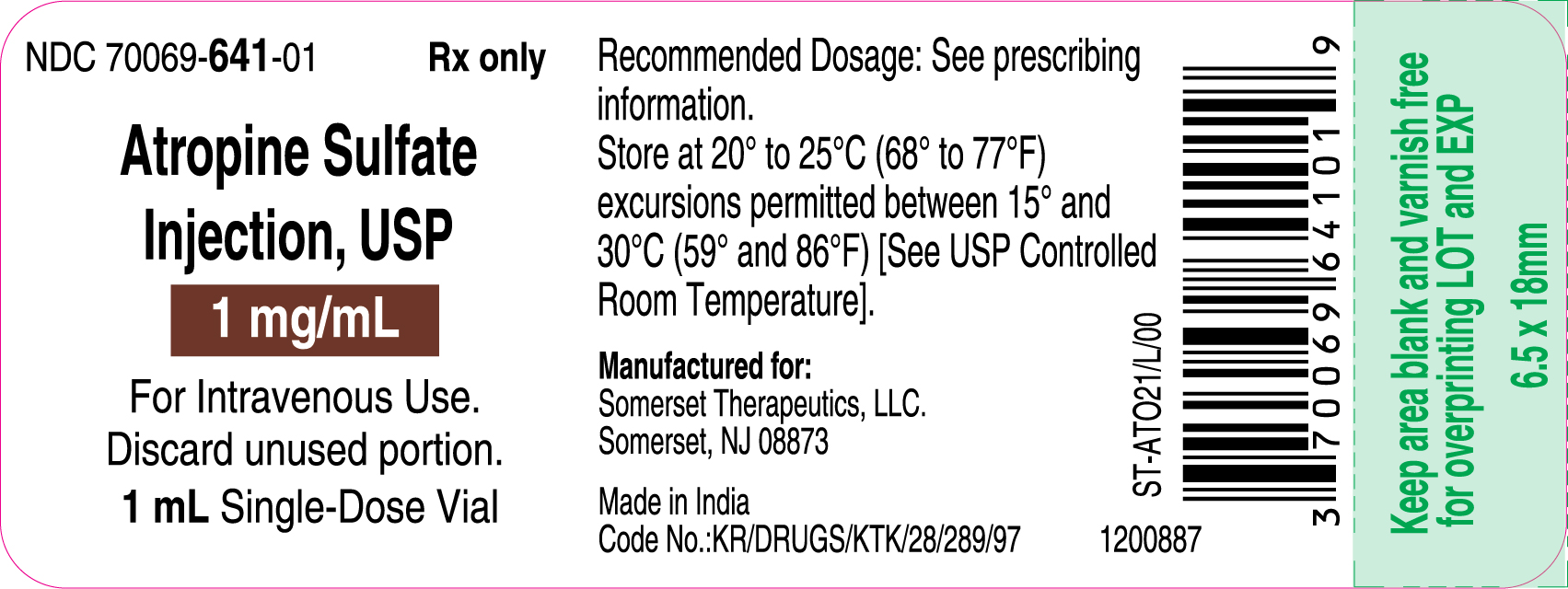
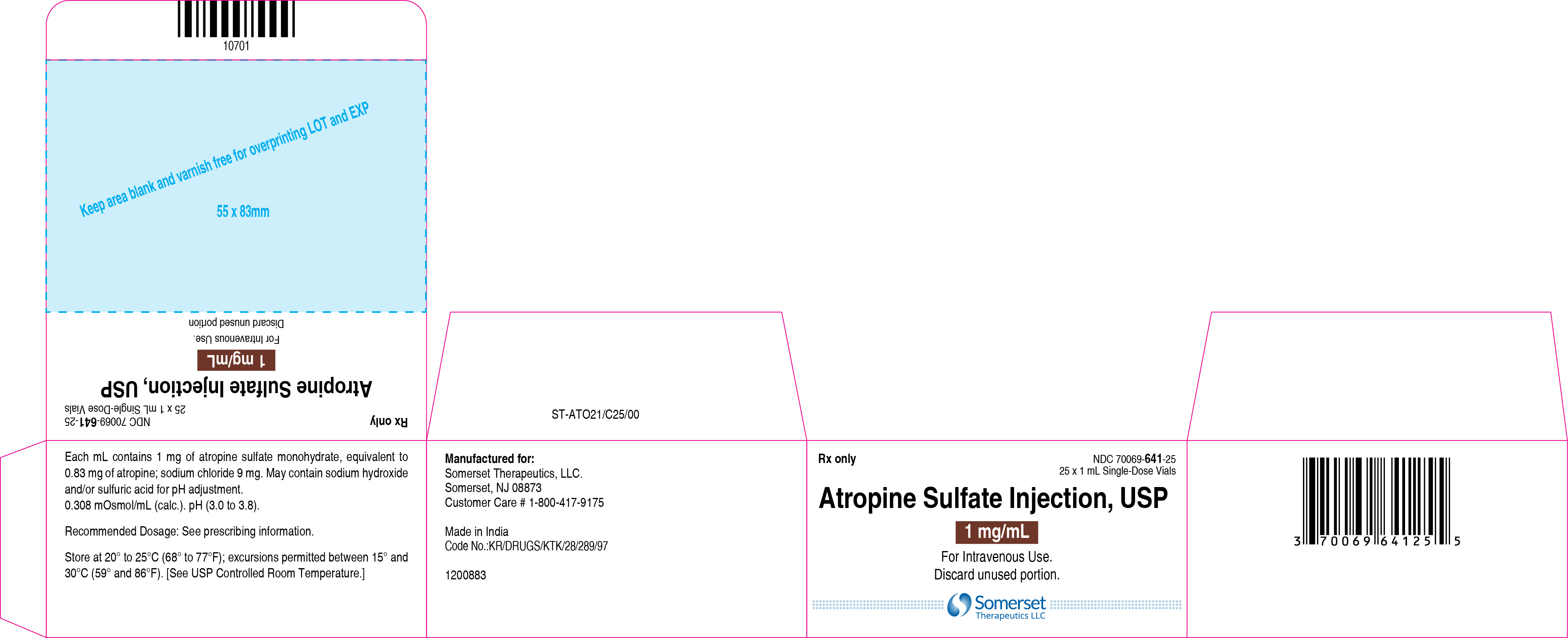
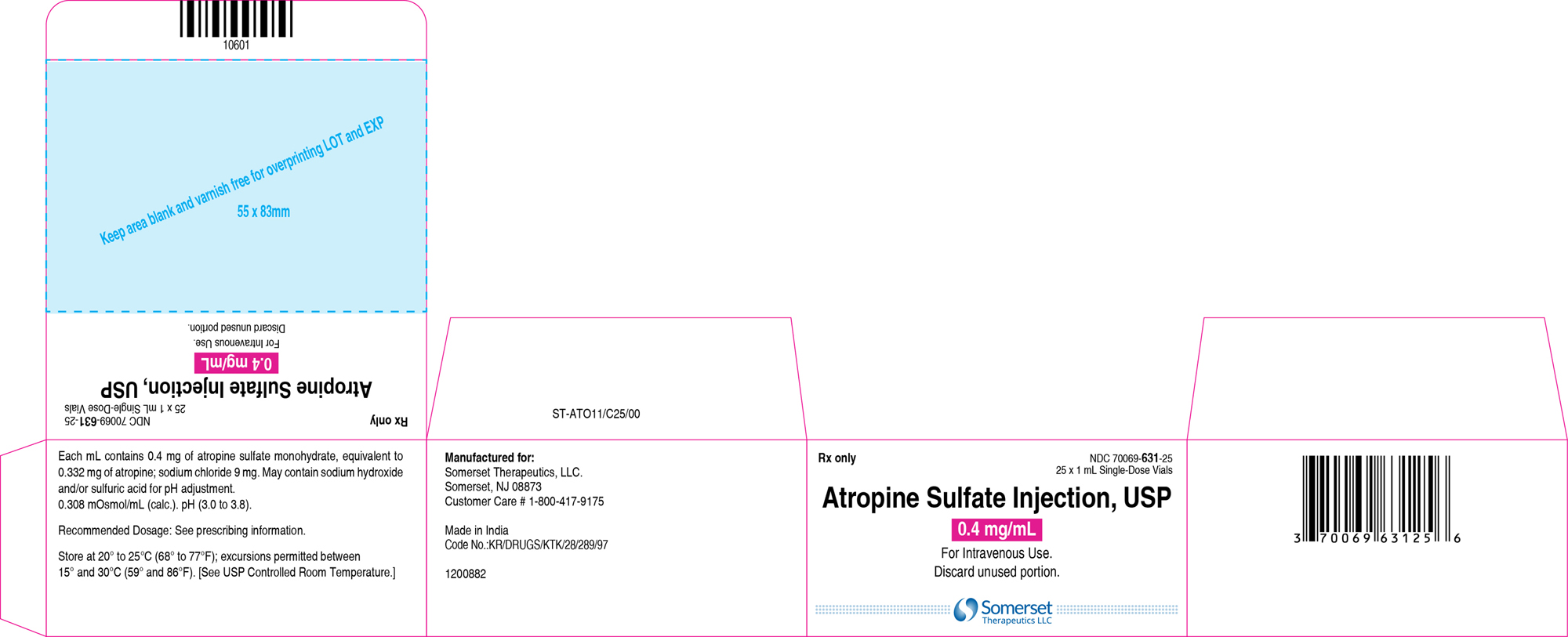
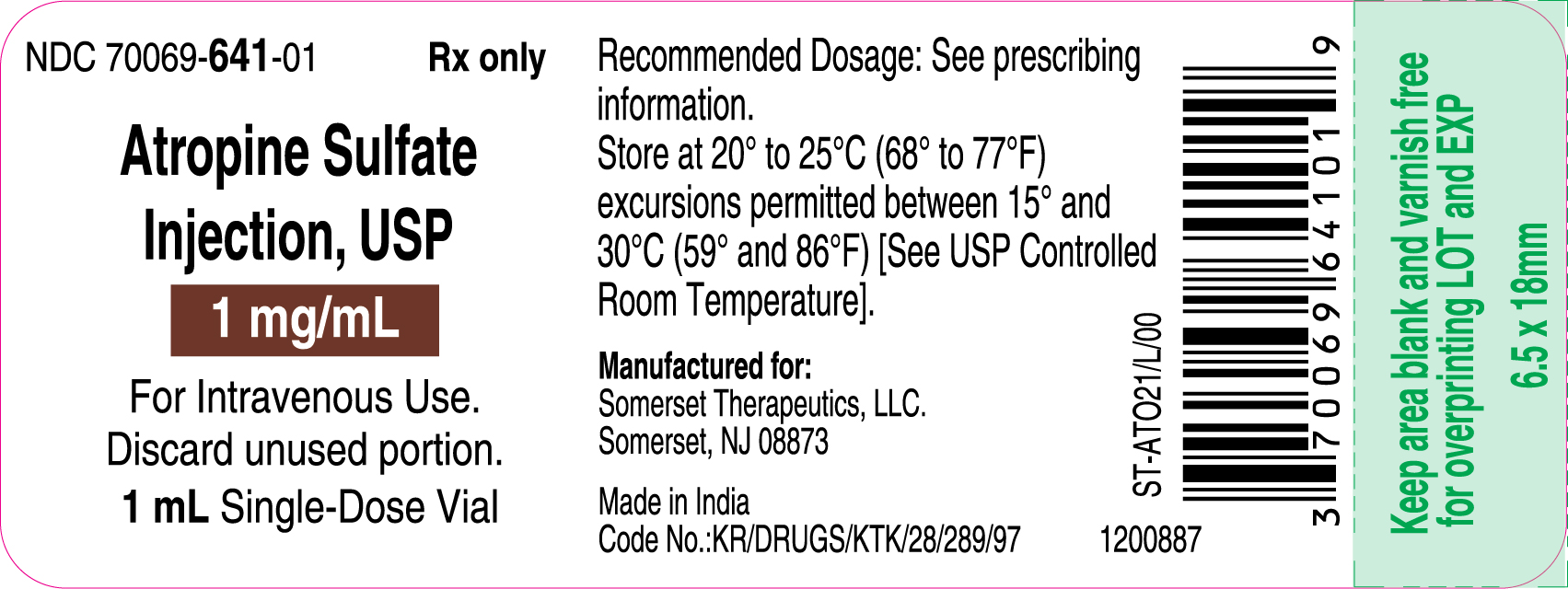
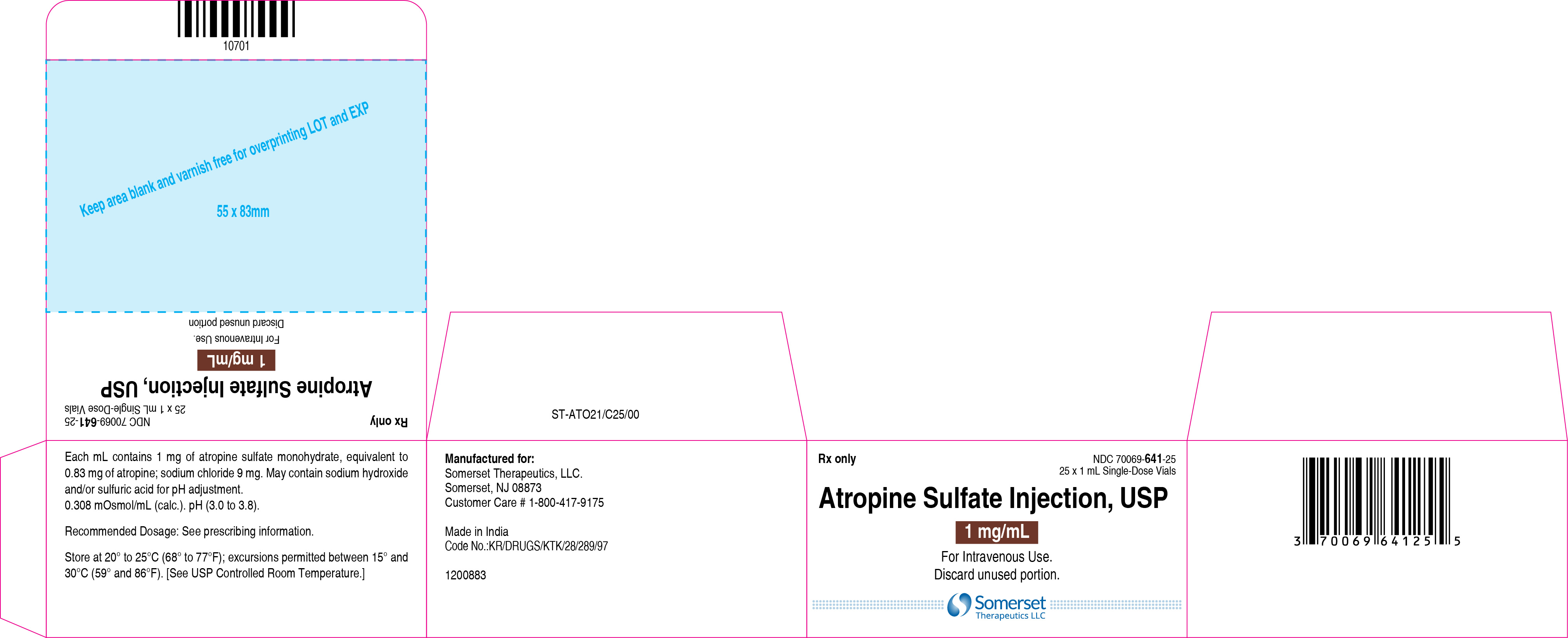
3 DOSAGE FORMS AND STRENGTHSInjection: supplied as a clear, colorless solution in a 1 mL glass vial in the following concentrations; 0.4 mg/mL: containing 0.4 mg of atropine sulfate monohydrate equivalent to 0.332 mg of ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Tachycardia - When the recurrent use of atropine is essential in patients with coronary artery disease, the total dose should be restricted to 2 to 3 mg (maximum 0.03 to 0.04 mg/kg) to avoid ...
-
6 ADVERSE REACTIONSThe following adverse reactions have been identified during post-approval use of atropine sulfate. Because these reactions are reported voluntarily from a population of uncertain size, it is not ...
-
7 DRUG INTERACTIONS7.1 Mexiletine - Atropine Sulfate Injection decreased the rate of mexiletine absorption without altering the relative oral bioavailability; this delay in mexiletine absorption was reversed by ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are risks to the mother and fetus associated with untreated severe or life-threatening muscarinic events (see Clinical Considerations). Available data from ...
-
10 OVERDOSAGEExcessive dosing may cause palpitation, dilated pupils, difficulty in swallowing, hot dry skin, thirst, dizziness, restlessness, tremor, fatigue and ataxia. Toxic doses lead to restlessness and ...
-
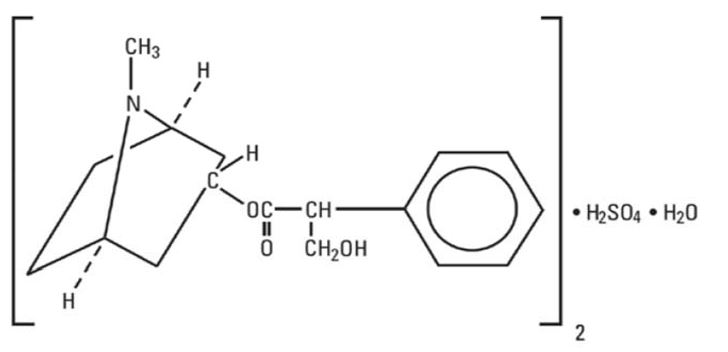
11 DESCRIPTIONAtropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate in water for injection with sodium chloride sufficient to render the solution ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Atropine is an antimuscarinic agent since it antagonizes the muscarine-like actions of acetylcholine and other choline esters. Atropine inhibits the muscarinic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies have not been performed to evaluate the carcinogenic or mutagenic potential of atropine or its potential to affect fertility ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAtropine Sulfate Injection, USP 0.4 and 1 mg/mL are supplied in 1 mL, single-dose glass vials as follows: Table 2: How Supplied - Concentration (mg/mL) Package Size - NDC ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Somerset Therapeutics, LLC - Somerset, NJ 08873 - Customer Care # 1-800-417-9175 - Made in India - Code No.: KR/DRUGS/KTK/28/289/97 - 1200885 - ST-ATO/P/00
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELContainer Label – 0.4 mg/mL - Carton Label 25's Pack – 0.4 mg/mL - Container Label – 1 mg/mL - Carton Label 25's Pack – 1 mg/mL
-
INGREDIENTS AND APPEARANCEProduct Information