Label: INDOCYANINE GREEN- indocyanine green and water kit
- NDC Code(s): 0409-4887-17, 70100-424-02
- Packager: Renew Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 13, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INDOCYANINE GREEN for injection safely and effectively. See full prescribing information for INDOCYANINE GREEN for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Indocyanine Green for injection is indicated: 1.1 Visualization of Vessels, Blood Flow and Tissue Perfusion - Indocyanine Green is indicated for fluorescence imaging of vessels (micro- and ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Indicator-Dilution Studies - Dosing - Adults: The recommended dose of Indocyanine Green for a single image sequence for visualization of vessels, blood flow and tissue perfusion in ...
-
3 DOSAGE FORMS AND STRENGTHS For injection: 25 mg of indocyanine green as a sterile, lyophilized, green powder for reconstitution provided in a 25 mL single-patient-use vial.
-
4 CONTRAINDICATIONS Indocyanine Green is contraindicated in patients with a history of hypersensitivity to indocyanine green. Reactions have included anaphylaxis [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity Reactions - Hypersensitivity reactions including anaphylaxis, urticaria and deaths due to anaphylaxis have been reported following intravenous administration of Indocyanine ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Hypersensitivity Reactions [see Warnings and Precautions (5.1)]. The following adverse reactions ...
-
7 DRUG INTERACTIONS Interference with Thyroid Radioactive Iodine Uptake Studies - Because Indocyanine Green contains sodium iodide, the iodine-binding capacity of thyroid tissue may be reduced for at least one week ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of Indocyanine Green in pregnant women. Available data from a very small number of scientific literature ...
-
11 DESCRIPTION Indocyanine Green for injection is an optical imaging agent for intravenous or interstitial use. Each vial contains 25 mg of indocyanine green with not more than 5% sodium iodide as a sterile ...
-
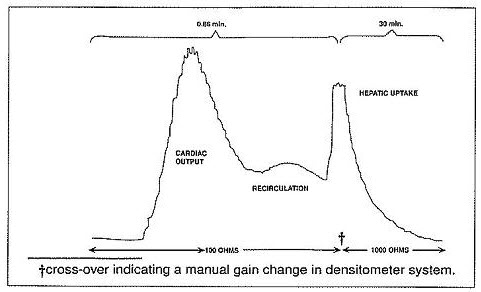
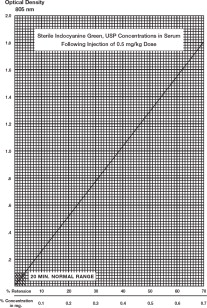
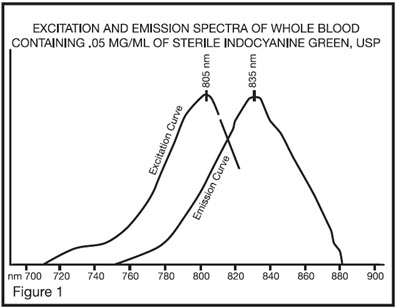
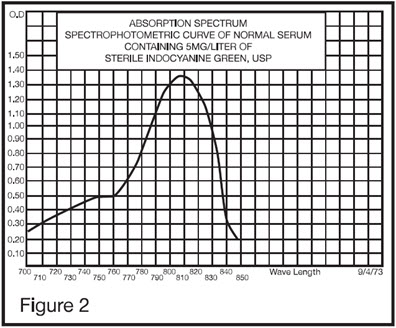
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - When bound to proteins in plasma or in lymph fluid, indocyanine green absorbs light in the near-infrared region with peak absorption at 805 nm and emits fluorescence ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been performed to evaluate the potential for carcinogenicity, mutagenicity, or impairment of fertility by indocyanine ...
-
14 CLINICAL STUDIES 14.1 Lymphatic Mapping of Cervical and Uterine Cancer - The effectiveness of Indocyanine Green for fluorescence imaging of lymph nodes and delineation of lymphatic vessels during lymphatic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - Indocyanine Green for injection is supplied as a kit (NDC 70100-424-02) containing the following: • Six 25 mL single-patient-use vials of Indocyanine Green (25 mg each) as a ...
-
17 PATIENT COUNSELING INFORMATION Hypersensitivity Reactions - Advise patients to seek medical attention for reactions following injection of Indocyanine Green such as difficulty breathing, swollen tongue or throat, skin reactions ...
-
Manufactured by: Patheon Italia S.p.A. 20900 Monza (MB), ITALY - or - LYOCONTRACT GmbH - 38871 Ilsenburg, GERMANY - Distributed by: Diagnostic Green LLC - Farmington Hills, Ml 48331 - Sterile Water for ...
-
PRINCIPAL DISPLAY PANEL - Vial Vial Label - NDC 70100-424-01 - Rx only Sterile - Indocyanine Green - for Injection, USP - 25 mg/Vial - Single-Patient-Use - For Intravenous or Interstitial Use - After reconstitution, use within 6 ...
-
PRINCIPAL DISPLAY PANEL - STERILE WATER VIAL Sterile Water Label - 10 mL Single-dose - Sterile Water - for Injection, USP - FOR DRUG DILUENT USE - Rx only NDC 0409-4887-17 - Contains no antimicrobial or other added - substance ...
-
PRINCIPAL DISPLAY PANEL - Carton Carton Label - NDC 70100-424-02 Rx Only - Sterile - Indocyanine Green - for Injection, USP - 25 mg/Vial - Single-Patient-Use For Intravenous or Interstitial Use - Distributed ...
-
INGREDIENTS AND APPEARANCEProduct Information