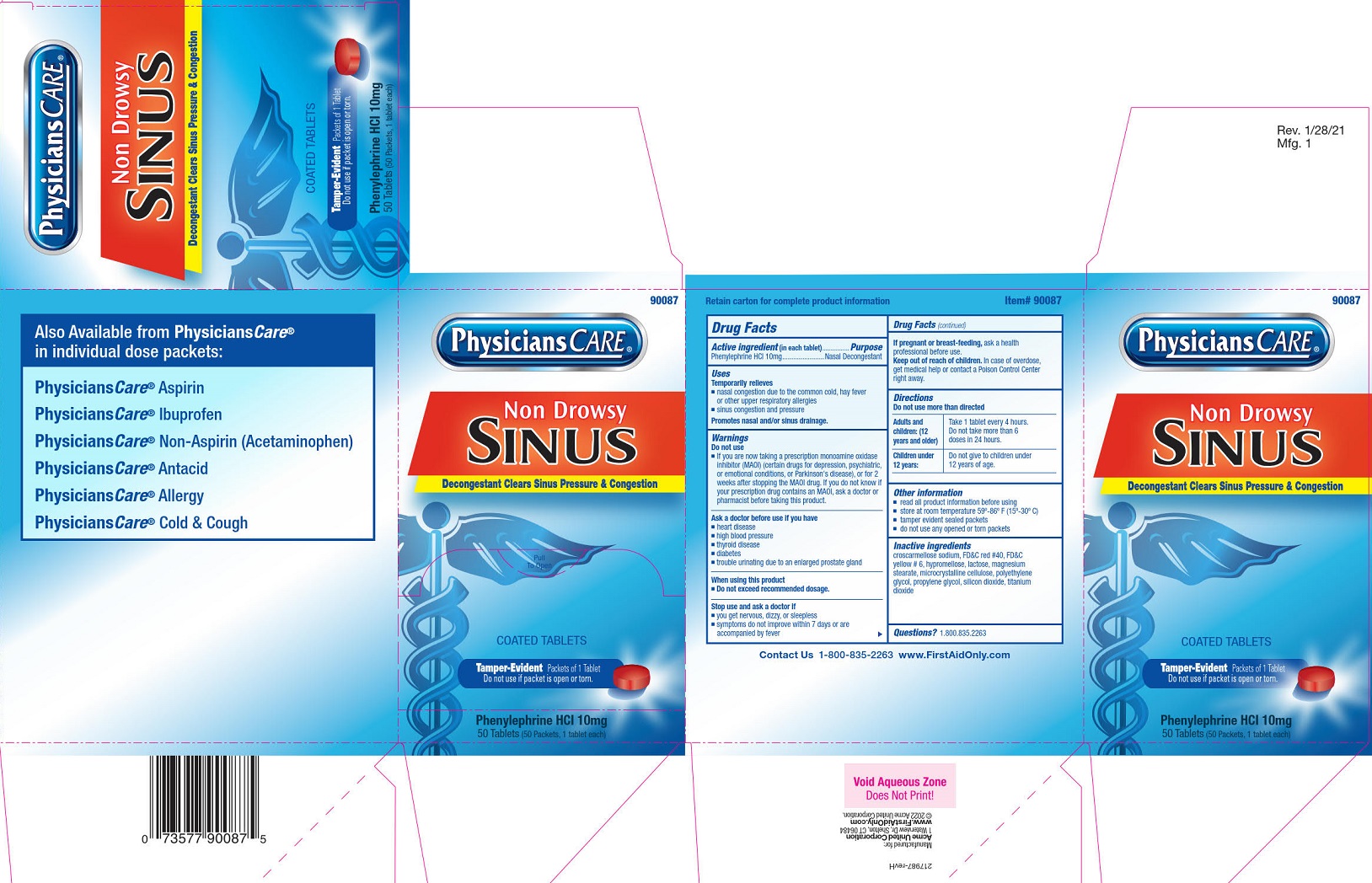
Label: PHYSICIANSCARE SINUS NON-DROWSY- phenylephrine hcl tablet, film coated
- NDC Code(s): 0924-0133-00, 0924-0133-01, 0924-0133-03
- Packager: Acme United Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DO NOT USE
Do not use
■ If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INFORMATION FOR PATIENTS
- INACTIVE INGREDIENT
- Questions or comments?
- Physicians Care Sinus Label
-
INGREDIENTS AND APPEARANCE
PHYSICIANSCARE SINUS NON-DROWSY
phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-0133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 2000 (UNII: HAF0412YIT) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red (red) Score no score Shape ROUND (ROUND) Size 7mm Flavor Imprint Code 272 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-0133-01 50 in 1 CARTON 01/29/2012 1 NDC:0924-0133-00 1 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:0924-0133-03 10 in 1 CARTON 01/29/2012 2 NDC:0924-0133-00 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/29/2012 Labeler - Acme United Corporation (001180207)