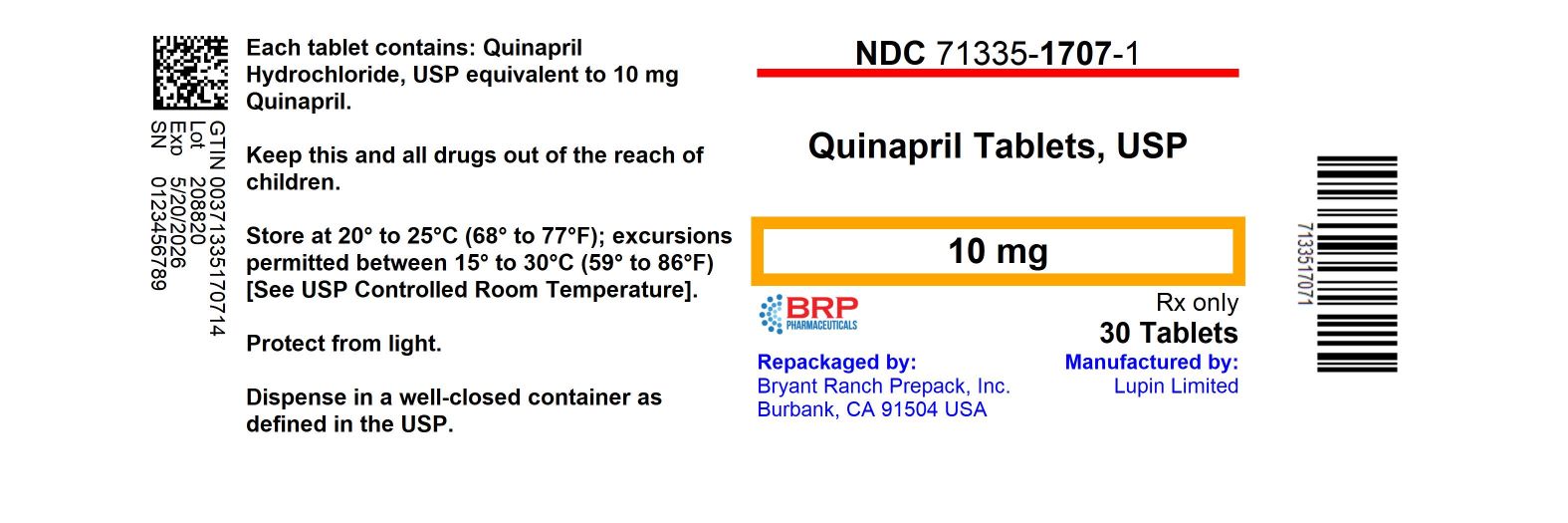
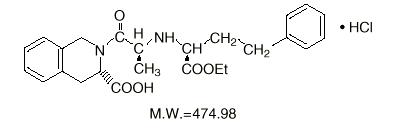
General
-
Impaired renal function
-
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients ...
General
Impaired renal function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe heart failure whose renal function may depend on the activity of the renin- angiotensin-aldosterone system, treatment with ACE inhibitors, including quinapril, may be associated with oliguria and/or progressive azotemia and rarely acute renal failure and/or death.
In clinical studies in hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine have been observed in some patients following ACE inhibitor therapy. These increases were almost always reversible upon discontinuation of the ACE inhibitor and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy.
Some patients with hypertension or heart failure with no apparent preexisting renal vascular disease have developed increases in blood urea and serum creatinine, usually minor and transient, especially when quinapril has been given concomitantly with a diuretic. This is more likely to occur in patients with preexisting renal impairment. Dosage reduction and/or discontinuation of any diuretic and/or quinapril may be required.
Evaluation of patients with hypertension or heart failure should always include assessment of renal function (see DOSAGE AND ADMINISTRATION).
Hyperkalemia
In clinical trials, hyperkalemia (serum potassium ≥5.8 mmol/L) occurred in approximately 2% of patients receiving quinapril. In most cases, elevated serum potassium levels were isolated values which resolved despite continued therapy. Less than 0.1% of patients discontinued therapy due to hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of other drugs that raise serum potassium levels. Monitor serum potassium in such patients (see PRECAUTIONS, Drug Interactions).
Cough
Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent non-productive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Surgery/anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, quinapril will block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Information for Patients
Pregnancy
Tell female patients of childbearing age about the consequences of exposure to quinapril during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible.
Angioedema
Angioedema, including laryngeal edema can occur with treatment with ACE inhibitors, especially following the first dose. Advise patients and tell them to immediately report any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to stop taking the drug until they have consulted with their physician (see WARNINGS).
Symptomatic hypotension
Caution patients that lightheadedness can occur, especially during the first few days of quinapril therapy, and that it should be reported to a physician. If actual syncope occurs, tell patients to temporarily discontinue the drug until they have consulted with their physician (see WARNINGS).
Caution all patients that inadequate fluid intake or excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure because of reduction in fluid volume, with the same consequences of lightheadedness and possible syncope.
Tell patients planning to undergo any surgery and/or anesthesia to inform their physician that they are taking an ACE inhibitor.
Hyperkalemia
Tell patients not to use potassium supplements or salt substitutes containing potassium without consulting their physician (see PRECAUTIONS).
Neutropenia
Tell patients to promptly report any indication of infection (eg, sore throat, fever) which could be a sign of neutropenia.
NOTE: As with many other drugs, certain advice to patients being treated with quinapril is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Drug Interactions
Concomitant diuretic therapy
As with other ACE inhibitors, patients on diuretics, especially those on recently instituted diuretic therapy, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with quinapril. The possibility of hypotensive effects with quinapril may be minimized by either discontinuing the diuretic or cautiously increasing salt intake prior to initiation of treatment with quinapril. If it is not possible to discontinue the diuretic, the starting dose of quinapril should be reduced (see DOSAGE AND ADMINISTRATION).
Agents increasing serum potassium
Coadministration of quinapril with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
Tetracycline and other drugs that interact with magnesium
Simultaneous administration of tetracycline with quinapril reduced the absorption of tetracycline by approximately 28% to 37%, possibly due to the high magnesium content in quinapril tablets. This interaction should be considered if coprescribing quinapril and tetracycline or other drugs that interact with magnesium.
Lithium
Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving concomitant lithium and ACE inhibitor therapy. These drugs should be coadministered with caution and frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, it may increase the risk of lithium toxicity.
Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting, and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy.
Non-steroidal anti-inflammatory agents including selective cyclooxygenase-2 inhibitors (COX-2 inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including quinapril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving quinapril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including quinapril may be attenuated by NSAIDs.
Agents that inhibit mTOR or other drugs known to cause angioedema
Patients taking concomitant mTOR inhibitor (e.g., temsirolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema.
Other agents
Drug interaction studies of quinapril with other agents showed:
- Multiple dose therapy with propranolol or cimetidine has no effect on the pharmacokinetics of single doses of quinapril.
- The anticoagulant effect of a single dose of warfarin (measured by prothrombin time) was not significantly changed by quinapril coadministration twice-daily.
- Quinapril treatment did not affect the pharmacokinetics of digoxin.
- No pharmacokinetic interaction was observed when single doses of quinapril and hydrochlorothiazide were administered concomitantly.
- Co-administration of multiple 10 mg doses of atorvastatin with 80 mg of quinapril resulted in no significant change in the steady-state pharmacokinetic parameters of atorvastatin.
Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on quinapril and other agents that affect the RAS.
Do not co-administer aliskiren with quinapril in patients with diabetes. Avoid concomitant use of aliskiren with quinapril in patients with renal impairment (GFR <60 mL/min/1.73 m2).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Quinapril hydrochloride was not carcinogenic in mice or rats when given in doses up to 75 or 100 mg/kg/day (50 to 60 times the maximum human daily dose, respectively, on an mg/kg basis and 3.8 to 10 times the maximum human daily dose when based on an mg/m2 basis) for 104 weeks. Female rats given the highest dose level had an increased incidence of mesenteric lymph node hemangiomas and skin/subcutaneous lipomas. Neither quinapril nor quinaprilat were mutagenic in the Ames bacterial assay with or without metabolic activation. Quinapril was also negative in the following genetic toxicology studies: in vitro mammalian cell point mutation, sister chromatid exchange in cultured mammalian cells, micronucleus test with mice, in vitro chromosome aberration with V79 cultured lung cells, and in an in vivo cytogenetic study with rat bone marrow. There were no adverse effects on fertility or reproduction in rats at doses up to 100 mg/kg/day (60 and 10 times the maximum daily human dose when based on mg/kg and mg/m2, respectively).
Nursing Mothers
Because quinapril is secreted in human milk, caution should be exercised when this drug is administered to a nursing woman.
Pediatric Use
Neonates with a history of in utero exposure to quinapril:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Removal of quinapril, which crosses the placenta, from the neonatal circulation is not significantly accelerated by these means.
The safety and effectiveness of quinapril in pediatric patients have not been established.
Geriatric Use
Clinical studies of quinapril did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly patients exhibited increased area under the plasma concentration time curve and peak levels for quinaprilat compared to values observed in younger patients; this appeared to relate to decreased renal function rather than to age itself.
Close