Label: EPINEPHRINE injection, solution, concentrate
- NDC Code(s): 63323-696-02, 63323-696-25
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPINEPHRINE INJECTION safely and effectively. See full prescribing information for EPINEPHRINE INJECTION. EPINEPHRINE injection ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
1.1 Hypotension associated with Septic Shock - Epinephrine Injection USP, 1 mg/mL is indicated to increase mean arterial blood pressure in adult patients with hypotension associated with septic ...
-
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations - Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the solution is colored or ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection solution: 1 mg/mL epinephrine as a sterile solution in a 1 mL single-dose glass vial, marked Epinephrine Injection USP, 1 mg/mL.
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypertension - When Epinephrine Injection is administered intravenously, titrate the infusion while monitoring vital signs. Invasive arterial blood pressure monitoring and central venous ...
-
6 ADVERSE REACTIONS
6.1 Adverse Reactions associated with Epinephrine Infusion (for Hypotension associated with Septic Shock) The following adverse reactions associated with the infusion of epinephrine were ...
-
7 DRUG INTERACTIONS
Drugs antagonizing pressor effects of epinephrine - α-blockers, such as phentolamine - Vasodilators, such as nitrates - Diuretics - Antihypertensives - Ergot alkaloids - Drugs potentiating pressor ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Prolonged experience with epinephrine use in pregnant women over several decades, based on published literature, does not identify a drug-associated risk of ...
-
10 OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in ...
-
11 DESCRIPTION
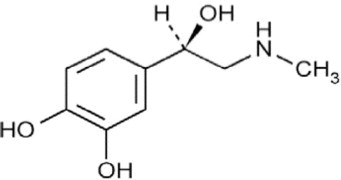
Epinephrine Injection, USP is a clear, colorless, sterile solution containing 1 mg/mL epinephrine, packaged as a 1 mL solution in a 1 mL single-dose vial. Each mL of Epinephrine Injection, USP ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Epinephrine acts on both alpha (α)- and beta (β)-adrenergic receptors. The mechanism of the rise in blood pressure is 3-fold: a direct myocardial stimulation that ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted. Epinephrine and other catecholamines ...
-
14 CLINICAL STUDIES
14.1 Hypotension associated with Septic Shock - Fourteen clinical studies from the literature documented that epinephrine increases the mean arterial pressure (MAP) in patients with hypotension ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Epinephrine Injection, USP is supplied as follows: Product CodeUnit of SaleStrengthEach - 696125 - NDC 63323-696-25 - Unit of 25 - 1 mg/mL - NDC 63323-696-02 - 1 mL Single-Dose Vial - Epinephrine ...
-
17 PATIENT COUNSELING INFORMATION
Advise patients or their caregivers about common adverse reactions associated with the use of epinephrine, including an increase in heart rate, the sensation of a more forceful heartbeat ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Epinephrine Injection, USP 1 mL Single-Dose Vial Label - NDC 63323-696-02 - Epinephrine - Injection, USP - 1 mg/mL - Dilute before Intravenous - and Intraocular ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Epinephrine Injection, USP 1 mL Tray Label - NDC 63323-696-25 - Epinephrine Injection, USP - 1 mg/mL - Dilute before Intravenous and Intraocular use. For ...
-
INGREDIENTS AND APPEARANCEProduct Information