Label: SILDENAFIL CITRATE tablet
- NDC Code(s): 0904-6671-04, 0904-6671-06
- Packager: Major Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 13668-185
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SILDENAFIL TABLETS safely and effectively. See full prescribing information for SILDENAFIL TABLETS.
SILDENAFIL tablets, for oral use
Initial U.S. Approval: 1998INDICATIONS AND USAGE
Adults
Sildenafil citrate is a phosphodiesterase-5 (PDE-5) inhibitor indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening. (1)DOSAGE AND ADMINISTRATION
• Adults: 20 mg three times a day. (2.1)
DOSAGE FORMS AND STRENGTHS
- •
- Tablets: 20 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Vasodilation effects may be more common in patients with hypotension or on antihypertensive therapy. (5.1)
- •
- Use in pulmonary veno-occlusive disease (PVOD) may cause pulmonary edema and is not recommended. (5.2)
- •
- Hearing or visual impairment: Seek medical attention if sudden decrease or loss of vision or hearing occurs. (5.4, 5.5)
- •
- Pulmonary hypertension (PH) secondary to sickle cell disease: Sildenafil citrate may cause serious vaso-occlusive crises. (5.8)
ADVERSE REACTIONS
DRUG INTERACTIONS
• Use with strong CYP3A inhibitors: Not recommended. (7, 12.3)
• Concomitant PDE-5 inhibitors: Avoid use with Viagra® or other PDE-5 inhibitors. (5.6)Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Worsening Pulmonary Vascular Occlusive Disease
5.3 Epistaxis
5.4 Visual Loss
5.5 Hearing Loss
5.6 Combination with other PDE-5 inhibitors
5.7 Priapism
5.8 Vaso-occlusive Crisis in Patients with Pulmonary Hypertension Secondary to Sickle Cell Disease
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Impairment
8.7 Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Adults
Sildenafil tablets are indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
Oral Dosage
The recommended dosage of sildenafil tablets are 20 mg three times a day. [see Clinical Studies (14)]
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Sildenafil citrate is contraindicated in patients with:
- •
- Concomitant use of organic nitrates in any form, either regularly or intermittently, because of the greater risk of hypotension [see Warnings and Precautions ( 5.1)] .
- •
- Concomitant use of riociguat, a guanylate cyclase stimulator. Phosphodiesterase-5 (PDE5) inhibitors, including sildenafil, may potentiate the hypotensive effects of riociguat.
- •
- Known hypersensitivity to sildenafil or any component of the tablet. Hypersensitivity, including anaphylactic reaction, anaphylactic shock and anaphylactoid reaction, has been reported in association with the use of sildenafil.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Sildenafil citrate has vasodilatory properties, resulting in mild and transient decreases in blood pressure. Before prescribing sildenafil tablets, carefully consider whether patients with certain underlying conditions could be adversely affected by such vasodilatory effects (e.g., patients on antihypertensive therapy or with resting hypotension [blood pressure less than 90/50], fluid depletion, severe left ventricular outflow obstruction, or autonomic dysfunction). Monitor blood pressure when co-administering blood pressure lowering drugs with sildenafil citrate.
5.2 Worsening Pulmonary Vascular Occlusive Disease
Pulmonary vasodilators may significantly worsen the cardiovascular status of patients with pulmonary veno-occlusive disease (PVOD). Since there are no clinical data on administration of sildenafil citrate to patients with veno-occlusive disease, administration of sildenafil citrate to such patients is not recommended. Should signs of pulmonary edema occur when sildenafil citrate is administered, consider the possibility of associated PVOD.
5.3 Epistaxis
The incidence of epistaxis was 13% in patients taking sildenafil citrate with PAH secondary to CTD. This effect was not seen in idiopathic PAH (sildenafil 3%, placebo 2%) patients. The incidence of epistaxis was also higher in sildenafil-treated patients with a concomitant oral vitamin K antagonist (9% versus 2% in those not treated with concomitant vitamin K antagonist).
The safety of sildenafil citrate is unknown in patients with bleeding disorders or active peptic ulceration.
5.4 Visual Loss
When used to treat erectile dysfunction, non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported postmarketing in temporal association with the use of PDE-5 inhibitors, including sildenafil. Most patients had underlying anatomic or vascular risk factors for developing NAION, including low cup to disc ratio (“crowded disc”).
Advise patients to seek immediate medical attention in the event of a sudden loss of vision in one or both eyes while taking sildenafil citrate.
There are no controlled clinical data on the safety or efficacy of sildenafil citrate in patients with retinitis pigmentosa, a minority of whom have genetic disorders of retinal phosphodiesterases. Therefore, use of sildenafil citrate in patients with retinitis pigmentosa is not recommended.
5.5 Hearing Loss
Cases of sudden decrease or loss of hearing, which may be accompanied by tinnitus and dizziness, have been reported in temporal association with the use of PDE-5 inhibitors, including sildenafil citrate. In some of the cases, medical conditions and other factors were reported that may have played a role. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of sildenafil citrate, to the patient's underlying risk factors for hearing loss, a combination of these factors, or to other factors.
Advise patients to seek prompt medical attention in the event of sudden decrease or loss of hearing while taking PDE-5 inhibitors, including sildenafil tablets.
5.6 Combination with other PDE-5 inhibitors
Sildenafil is also marketed as VIAGRA®. The safety and efficacy of combinations of sildenafil tablets with VIAGRA or other PDE-5 inhibitors have not been studied. Inform patients taking sildenafil tablets not to take VIAGRA or other PDE-5 inhibitors.
5.7 Priapism
Use sildenafil citrate with caution in patients with anatomical deformation of the penis (e.g., angulation, cavernosal fibrosis, or Peyronie's disease) or in patients who have conditions, which may predispose them to priapism (e.g., sickle cell anemia, multiple myeloma, or leukemia). In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism (painful erection greater than 6 hours in duration) is not treated immediately, penile tissue damage and permanent loss of potency could result.
5.8 Vaso-occlusive Crisis in Patients with Pulmonary Hypertension Secondary to Sickle Cell Disease
In a small, prematurely terminated study of patients with pulmonary hypertension (PH) secondary to sickle cell disease, vaso-occlusive crises requiring hospitalization were more commonly reported by patients who received sildenafil citrate than by those randomized to placebo. The effectiveness and safety of sildenafil citrate in the treatment of PH secondary to sickle cell disease has not been established.
-
6 ADVERSE REACTIONS
The following serious adverse events are discussed elsewhere in the labeling:
- •
- Hypotension [see Warnings and Precautions (5.1)]
- •
- Vision Loss [see Warnings and Precautions (5.4)]
- •
- Hearing Loss [see Warnings and Precautions (5.5)]
- •
- Priapism [see Warnings and Precautions (5.7)]
- •
- Vaso-occlusive Crisis in Patients with Pulmonary Hypertension Secondary to Sickle Cell Disease [see Warnings and Precautions (5.8) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 12-week, placebo-controlled clinical study and an open-label extension study (SUPER-1) in 277 sildenafil citrate-treated adults with PAH (WHO Group I) [see Clinical Studies (14)] the adverse reactions that were reported by at least 10% of sildenafil citrate-treated patients in any dosing group, and were more frequent in sildenafil citrate-treated patients than in placebo-treated patients are shown in Table 1. Adverse reactions were generally transient and mild to moderate in nature. The overall frequency of discontinuation in sildenafil citrate-treated patients was 3% (20 mg and 40 mg three times a day) and 8% (80 mg three times a day). The overall frequency of discontinuation for placebo was 3%.
Table 1. Most Common Adverse Reactions in Patients Treated with sildenafil tablets 20 mg, 40 mg, 80 mg and Placebo three times per day in SUPER-1 (More Frequent in sildenafil-Treated Patients than Placebo-Treated Patients) Sildenafil tablets
20 mg
(n = 69)Sildenafil tablets
40 mg
(n = 67)Sildenafil tablets
80 mg
(n = 71)Placebo
(n = 70)
Headache
46%
42%
49%
39%
Flushing
10%
9%
16%
4%
Pain in Limb
7%
15%
9%
6%
Myalgia
7%
6%
14%
4%
Back Pain
13%
13%
9%
11%
Dyspepsia
13%
8%
13%
7%
Diarrhea
9%
12%
10%
6%
In a placebo-controlled fixed dose titration study (PACES-1) of sildenafil citrate (starting with recommended dose of 20 mg and increased to 40 mg and then 80 mg all three times a day) as an adjunct to intravenous epoprostenol in patients with PAH, no new safety issues were identified except for edema, which occurred in 25% of subjects in the combined sildenafil citrate + epoprostenol group compared with 13% of subjects in the epoprostenol group [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of sildenafil (marketed for both PAH and erectile dysfunction). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular Events
In postmarketing experience with sildenafil at doses indicated for erectile dysfunction, serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, pulmonary hemorrhage, and subarachnoid and intracerebral hemorrhages have been reported in temporal association with the use of the drug. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of sildenafil without sexual activity. Others were reported to have occurred hours to days after use concurrent with sexual activity. It is not possible to determine whether these events are related directly to sildenafil, to sexual activity, to the patient's underlying cardiovascular disease, or to a combination of these or other factors.
Nervous System
Seizure, seizure recurrence
Ophthalmologic
NAION [see Warnings and Precautions (5.4), Patient Counseling Information (17)].
-
7 DRUG INTERACTIONS
Nitrates
Concomitant use of sildenafil citrate with nitrates in any form is contraindicated [see Contraindications (4)] .
Strong CYP3A Inhibitors
Concomitant use of sildenafil citrate with strong CYP3A inhibitors is not recommended [see Clinical Pharmacology (12.3)].Moderate-to-Strong CYP3A Inducers
Concomitant use of sildenafil citrate with moderate-to-strong CYP3A inducers (such as bosentan) decreases the sildenafil exposure. Dose up-titration of sildenafil citrate may be needed when initiating treatment with moderate-to-strong CYP3A inducers. Reduce the dose of sildenafil tablets to 20 mg three times a day when discontinuing treatment with moderate-to-strong CYP3A inducers [see Clinical Pharmacology (12.3) and Clinical Studies (14)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data from randomized controlled trials, case-controlled trials, and case series do not report a clear association with sildenafil and major birth defects, miscarriage, or adverse maternal or fetal outcomes when sildenafil is used during pregnancy. There are risks to the mother and fetus from untreated pulmonary arterial hypertension (see Clinical Considerations). Animal reproduction studies conducted with sildenafil showed no evidence of embryo-fetal toxicity or teratogenicity at doses up to 32-and 65-times the recommended human dose (RHD) of 20 mg three times a day in rats and rabbits, respectively (see Data).The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women with untreated pulmonary arterial hypertension are at risk for heart failure, stroke, preterm delivery, and maternal and fetal death.Data
Animal Data
No evidence of teratogenicity, embryotoxicity, or fetotoxicity was observed in pregnant rats or rabbits dosed with sildenafil 200 mg/kg/day during organogenesis, a level that is, on a mg/m2 basis, 32-and 65-times, respectively, the recommended human dose (RHD) of 20 mg three times a day. In a rat pre-and postnatal development study, the no-observed-adverse-effect dose was 30 mg/kg/day (equivalent to 5-times the RHD on a mg/m2 basis).8.2 Lactation
Risk Summary
Limited published data from a case report describe the presence of sildenafil and its active metabolite in human milk. There is insufficient information about the effects of sildenafil on the breastfed infant and no information on the effects of sildenafil on milk production. Limited clinical data during lactation preclude a clear determination of the risk of sildenafil citrate to an infant during lactation.8.4 Pediatric Use
The safety and effectiveness of Sildenafil citrate have not been established in pediatric patients younger than 1 year of age.
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Clinical studies of sildenafil citrate did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)] .
8.6 Patients with Hepatic Impairment
No dose adjustment for mild to moderate impairment is required. Severe impairment has not been studied [see Clinical Pharmacology (12.3)] .
8.7 Patients with Renal Impairment
No dose adjustment is required (including severe impairment CLcr <30 mL/min) [see Clinical Pharmacology (12.3)] .
-
10 OVERDOSAGE
In studies with healthy volunteers of single doses up to 800 mg, adverse events were similar to those seen at lower doses but rates and severities were increased.
In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to accelerate clearance as sildenafil is highly bound to plasma proteins and it is not eliminated in the urine.
-
11 DESCRIPTION
Sildenafil tablets, USP, phosphodiesterase-5 (PDE-5) inhibitor, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type-5 (PDE-5). Sildenafil is also marketed as VIAGRA® for erectile dysfunction.
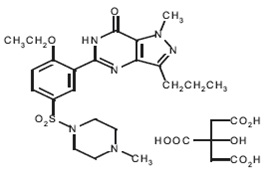
Sildenafil citrate, USP is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1 H-pyrazolo [4,3- d] pyrimidin-5-yl)-4- ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
Sildenafil citrate, USP is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7.
Sildenafil Tablets USP: Sildenafil citrate is formulated as white, film-coated round tablets for oral administration. Each tablet contains sildenafil citrate equivalent to 20 mg of sildenafil. In addition to the active ingredient, sildenafil citrate, each tablet contains the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate anhydrous, hypromellose, microcrystalline cellulose, sodium stearyl fumarate, titanium dioxide and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sildenafil is an inhibitor of cGMP specific PDE-5 in the smooth muscle of the pulmonary vasculature, where PDE-5 is responsible for degradation of cGMP. Sildenafil, therefore, increases cGMP within pulmonary vascular smooth muscle cells resulting in relaxation. In patients with PAH, this can lead to vasodilation of the pulmonary vascular bed and, to a lesser degree, vasodilatation in the systemic circulation.
Studies in vitro have shown that sildenafil is selective for PDE5. Its effect is more potent on PDE-5 than on other known phosphodiesterases (10-fold for PDE6, greater than 80-fold for PDE1, greater than 700-fold for PDE2, PDE3, PDE4, PDE7, PDE8, PDE9, PDE10, and PDE11). The approximately 4,000-fold selectivity for PDE5 versus PDE3 is important because PDE3 is involved in control of cardiac contractility. Sildenafil is only about 10-times as potent for PDE5 compared to PDE6, an enzyme found in the retina and involved in the phototransduction pathway of the retina. This lower selectivity is thought to be the basis for abnormalities related to color vision observed with higher doses or plasma levels [see Clinical Pharmacology (12.2)] .
In addition to pulmonary vascular smooth muscle and the corpus cavernosum, PDE5 is also found in other tissues including vascular and visceral smooth muscle and in platelets. The inhibition of PDE5 in these tissues by sildenafil may be the basis for the enhanced platelet anti-aggregatory activity of nitric oxide observed in vitro, and the mild peripheral arterial-venous dilatation in vivo.
12.2 Pharmacodynamics
Effects of Sildenafil Citrate on Hemodynamic Measures
Adults
Patients on all sildenafil citrate doses achieved a statistically significant reduction in mean pulmonary arterial pressure (mPAP) compared to those on placebo in a study with no background vasodilators [see SUPER-1 in Clinical Studies (14)]. Data on other hemodynamic measures for the sildenafil tablets 20 mg three times a day and placebo dosing regimens is displayed in Table 2. The relationship between these effects and improvements in 6-minute walk distance is unknown.
Table 2. Changes from Baseline in Hemodynamic Parameters at Week 12 [mean (95% CI)] for the Sildenafil Tablets 20 mg Three Times a Day and Placebo Group mPAP = mean pulmonary arterial pressure; PVR= pulmonary vascular resistance; SVR = systemic vascular resistance; RAP = right atrial pressure; CO = cardiac output; HR = heart rate * The number of patients per treatment group varied slightly for each parameter due to missing assessments. Placebo(n = 65)*
Sildenafil Tablets 20 mg (n = 65)*
mPAP (mmHg)
0.6 (-0.8, 2.0)
-2.1 (-4.3, 0.0)
PVR (dyn.s/cm5)
49 (-54, 153)
-122 (-217, -27)
SVR (dyn.s/cm5)
-78 (-197, 41)
-167 (-307, -26)
RAP (mmHg)
0.3 (-0.9, 1.5)
-0.8 (-1.9, 0.3)
CO (L/min)
-0.1 (-0.4, 0.2)
0.4 (0.1, 0.7)
HR (beats/min)
-1.3 (-4.1, 1.4)
-3.7 (-5.9, -1.4)
Effects of Sildenafil Citrate on Blood Pressure
Single oral doses of sildenafil 100 mg administered to healthy volunteers produced decreases in supine blood pressure (mean maximum decrease in systolic/diastolic blood pressure of 8/5 mmHg). The decrease in blood pressure was most notable approximately 1 to 2 hours after dosing and was not different from placebo at 8 hours. Similar effects on blood pressure were noted with 25 mg, 50 mg and 100 mg doses of sildenafil, therefore the effects are not related to dose or plasma levels within this dosage range. Larger effects were recorded among patients receiving concomitant nitrates [see Contraindications (4)] .
Single oral doses of sildenafil up to 100 mg in healthy volunteers produced no clinically relevant effects on electrocardiogram (ECG). After chronic dosing of 80 mg three times a day to patients with PAH, no clinically relevant effects on ECG were reported.
After chronic dosing of 80 mg three times a day sildenafil to healthy volunteers, the largest mean change from baseline in supine systolic and supine diastolic blood pressures was a decrease of 9.0 mmHg and 8.4 mmHg, respectively.
After chronic dosing of 80 mg three times a day sildenafil to patients with systemic hypertension, the mean change from baseline in systolic and diastolic blood pressures was a decrease of 9.4 mmHg and 9.1 mmHg, respectively.
After chronic dosing of 80 mg three times a day sildenafil to patients with PAH, lesser reductions than above in systolic and diastolic blood pressures were observed (a decrease in both of 2 mmHg).
Effects of Sildenafil Citrate on Vision
At single oral doses of 100 mg and 200 mg, transient dose-related impairment of color discrimination (blue/green) was detected using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina. An evaluation of visual function at doses up to 200 mg revealed no effects of sildenafil citrate on visual acuity, intraocular pressure, or pupillometry.
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
12.3 Pharmacokinetics
Absorption and Distribution
Sildenafil citrate is rapidly absorbed after oral administration, with a mean absolute bioavailability of 41% (25 to 63%). Maximum observed plasma concentrations are reached within 30 to 120 minutes (median 60 minutes) of oral dosing in the fasted state. When sildenafil tablet is taken with a high-fat meal, the rate of absorption is reduced, with a mean delay in T max of 60 minutes and a mean reduction in Cmax of 29%. The mean steady state volume of distribution (Vss) for sildenafil is 105 L, indicating distribution into the tissues. Sildenafil and its major circulating N-desmethyl metabolite are both approximately 96% bound to plasma proteins. Protein binding is independent of total drug concentrations.
Bioequivalence was established between the 20 mg tablet and the 10 mg/mL oral suspension when administered as a 20 mg single oral dose of sildenafil (as citrate).
Metabolism and Excretion
Sildenafil is cleared predominantly by the CYP3A (major route) and cytochrome P450 2C9 (CYP2C9, minor route) hepatic microsomal isoenzymes. The major circulating metabolite results from N-desmethylation of sildenafil, and is, itself, further metabolized. This metabolite has a phosphodiesterase selectivity profile similar to sildenafil and an in vitro potency for PDE-5 approximately 50% of the parent drug. In healthy volunteers, plasma concentrations of this metabolite are approximately 40% of those seen for sildenafil, so that the metabolite accounts for about 20% of sildenafil's pharmacologic effects. In patients with PAH, however, the ratio of the metabolite to sildenafil is higher. Both sildenafil and the active metabolite have terminal half-lives of about 4 hours.
After either oral or intravenous administration, sildenafil is excreted as metabolites predominantly in the feces (approximately 80% of the administered oral dose) and to a lesser extent in the urine (approximately 13% of the administered oral dose).
Population Pharmacokinetics
Age, gender, race, and renal and hepatic function were included as factors assessed in the population pharmacokinetic model to evaluate sildenafil pharmacokinetics in patients with PAH. The dataset available for the population pharmacokinetic evaluation contained a wide range of demographic data and laboratory parameters associated with hepatic and renal function. None of these factors had a significant impact on sildenafil pharmacokinetics in patients with PAH.
In patients with PAH, the average steady-state concentrations were 20 to 50% higher when compared to those of healthy volunteers. There was also a doubling of Cmin levels compared to healthy volunteers. Both findings suggest a lower clearance and/or a higher oral bioavailability of sildenafil in patients with PAH compared to healthy volunteers.
Pediatric Patients
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
Geriatric Patients
Healthy elderly volunteers (65 years or over) had a reduced clearance of sildenafil, resulting in approximately 84% and 107% higher plasma concentrations of sildenafil and its active N-desmethyl metabolite, respectively, compared to those seen in healthy younger volunteers (18 to 45 years). Due to age-differences in plasma protein binding, the corresponding increase in the AUC of free (unbound) sildenafil and its active N-desmethyl metabolite were 45% and 57%, respectively.
Renal Impairment
In volunteers with mild (CLcr = 50 to 80 mL/min) and moderate (CLcr = 30 to 49 mL/min) renal impairment, the pharmacokinetics of a single oral dose of sildenafil (50 mg) was not altered. In volunteers with severe (CLcr less than 30 mL/min) renal impairment, sildenafil clearance was reduced, resulting in approximately doubling of AUC and C max compared to age-matched volunteers with no renal impairment. In addition, N-desmethyl metabolite AUC and C max values were significantly increased 200% and 79%, respectively, in patients with severe renal impairment compared to patients with normal renal function.
Hepatic Impairment
In volunteers with mild to moderate hepatic cirrhosis (Child-Pugh class A and B), sildenafil clearance was reduced, resulting in increases in AUC (84%) and Cmax (47%) compared to age-matched volunteers with no hepatic impairment. Patients with severe hepatic impairment (Child-Pugh class C) have not been studied.
Drug Interaction Studies
In vitro studies
Sildenafil metabolism is principally mediated by the CYP3A (major route) and CYP2C9 (minor route) cytochrome P450 isoforms. Therefore, inhibitors of these isoenzymes may reduce sildenafil clearance and inducers of these isoenzymes may increase sildenafil clearance.
Sildenafil is a weak inhibitor of the cytochrome P450 isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A (IC50greater than 150 µM).
Sildenafil is not expected to affect the pharmacokinetics of compounds which are substrates of these CYP enzymes at clinically relevant concentrations.
In vivo studies
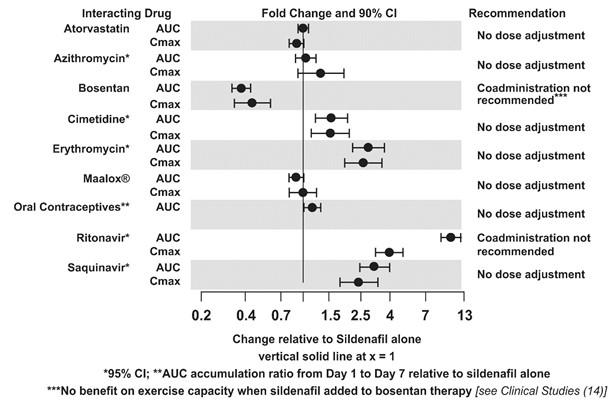
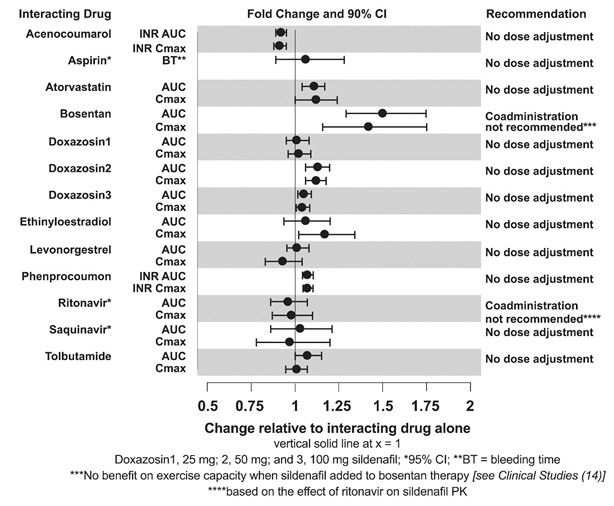
The effects of other drugs on sildenafil pharmacokinetics and the effects of sildenafil on the exposure to other drugs are shown in Figure 1 and Figure 2, respectively.
Figure 1. Effects of Other Drugs on Sildenafil Pharmacokinetics
Figure 2. Effects of Sildenafil on Other Drugs
CYP3A Inhibitors and Beta Blockers
Population pharmacokinetic analysis of data from patients in clinical trials indicated an approximately 30% reduction in sildenafil clearance when it was co-administered with mild/moderate CYP3A inhibitors and an approximately 34% reductions in sildenafil clearance when co-administered with beta-blockers. Sildenafil exposure at a dose of 80 mg three times a day without concomitant medication is shown to be 5-fold the exposure at a dose of 20 mg three times a day. This concentration range covers the same increased sildenafil exposure observed in specifically-designed drug interaction studies with CYP3A inhibitors (except for potent inhibitors such as ketoconazole, itraconazole, and ritonavir).
CYP3A4 Inducers Including Bosentan
Concomitant administration of strong CYP3A inducers is expected to cause substantial decreases in plasma levels of sildenafil.
Population pharmacokinetic analysis of data from patients in clinical trials indicated approximately 3-fold the sildenafil clearance when it was co-administered with mild CYP3A inducers.
Epoprostenol
The mean reduction of sildenafil (80 mg three times a day) bioavailability when co-administered with epoprostenol was 28%, resulting in about 22% lower mean average steady state concentrations. Therefore, the slight decrease of sildenafil exposure in the presence of epoprostenol is not considered clinically relevant. The effect of sildenafil on epoprostenol pharmacokinetics is not known.
No significant interactions were shown with tolbutamide (250 mg) or warfarin (40 mg), both of which are metabolized by CYP2C9.
Alcohol
Sildenafil (50 mg) did not potentiate the hypotensive effect of alcohol in healthy volunteers with mean maximum blood alcohol levels of 0.08%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Sildenafil was not carcinogenic when administered to rats for up to 24 months at 60 mg/kg/day, a dose resulting in total systemic exposure (AUC) to unbound sildenafil and its major metabolite 33- and 37-times, for male and female rats, respectively, the human exposure at the RHD of 20 mg three times a day. Sildenafil was not carcinogenic when administered to male and female mice for up to 21 and 18 months, respectively, at doses up to a maximally tolerated level of 10 mg/kg/day, a dose equivalent to the RHD on a mg/m2 basis.
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity.
There was no impairment of fertility in male or female rats given up to 60 mg sildenafil/kg/day, a dose producing a total systemic exposure (AUC) to unbound sildenafil and its major metabolite of 19- and 38-times for males and females, respectively, the human exposure at the RHD of 20 mg three times a day.
-
14 CLINICAL STUDIES
SUPER-1 (NCT00644605) - Sildenafil citrate monotherapy [20 mg, 40 mg, and 80 mg three times a day]
A randomized, double-blind, placebo-controlled study of sildenafil citrate (SUPER-1) was conducted in 277 patients with PAH (defined as a mean pulmonary artery pressure≥25 mmHg at rest with a pulmonary capillary wedge pressure<15 mmHg). Patients were predominantly WHO Functional Classes II-III. Allowed background therapy included a combination of anticoagulants, digoxin, calcium channel blockers, diuretics, and oxygen. The use of prostacyclin analogues, endothelin receptor antagonists, and arginine supplementation were not permitted. Patients who had failed to respond to bosentan were also excluded. Patients with left ventricular ejection fraction less than 45% or left ventricular shortening fraction less than 0.2 also were not studied.
Patients were randomized to receive placebo (n=70) or sildenafil tablet 20 mg (n = 69), 40 mg (n = 67) or 80 mg (n = 71) three times a day for a period of 12 weeks. They had either primary pulmonary hypertension (PPH) (63%), PAH associated with CTD (30%), or PAH following surgical repair of left-to-right congenital heart lesions (7%). The study population consisted of 25% men and 75% women with a mean age of 49 years (range: 18 to 81 years) and baseline 6-minute walk distance between 100 and 450 meters (mean 343).
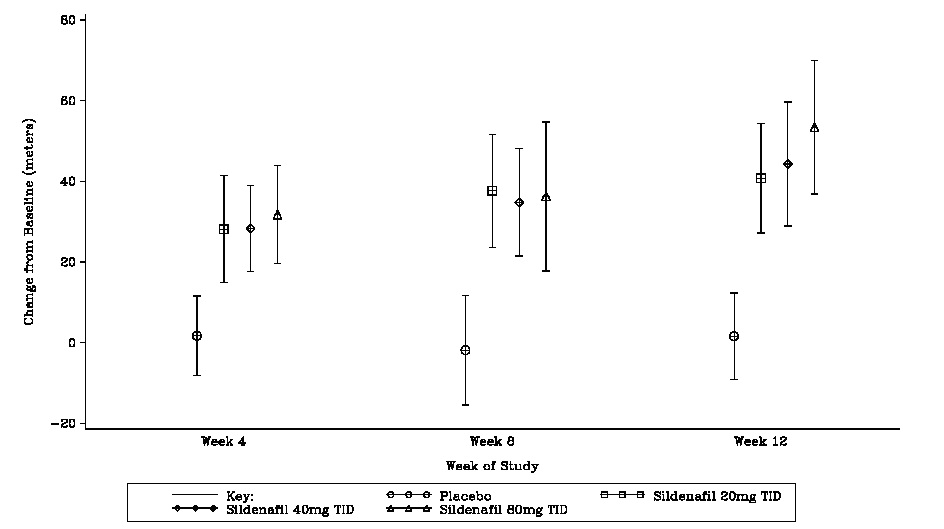
The primary efficacy endpoint was the change from baseline at Week 12 (at least 4 hours after the last dose) in the 6-minute walk distance. Placebo-corrected mean increases in walk distance of 45 to 50 meters were observed with all doses of sildenafil citrate. These increases were significantly different from placebo, but the sildenafil citrate dose groups were not different from each other (see Figure 3), indicating no additional clinical benefit from doses higher than 20 mg three times a day. The improvement in walk distance was apparent after 4 weeks of treatment and was maintained at Week 8 and Week 12.
Figure 3. Change from Baseline in 6-Minute Walk Distance (meters) at Weeks 4, 8, and 12 in SUPER-1: Mean (95% Confidence Interval)
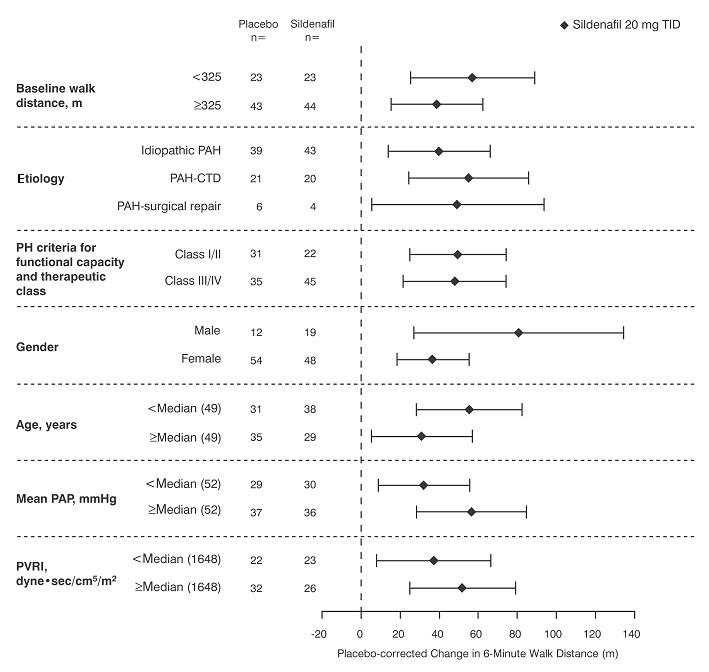
Figure 4 displays subgroup efficacy analyses in SUPER-1 for the change from baseline in 6-Minute Walk Distance at Week 12 including baseline walk distance, disease etiology, functional class, gender, age, and hemodynamic parameters.
Figure 4. Placebo-Corrected Change From Baseline in 6-Minute Walk Distance (meters) at Week 12 by Study Subpopulation in SUPER-1: Mean (95% Confidence Interval)
Key: PAH = pulmonary arterial hypertension; CTD = connective tissue disease; PH = pulmonary hypertension; PAP = pulmonary arterial pressure; PVRI = pulmonary vascular resistance index; TID = three times daily.
SUPER-2 (NCT00159887) Long-term Treatment of PAH
In a long-term follow-up of patients who were treated with sildenafil (n=277), K-M estimates of survival at 1, 2, and 3 years were 94%, 88% , and 79%, respectively. These uncontrolled observations do not allow comparison with a group not given sildenafil and cannot be used to determine the long term-effect of sildenafil on mortality.
PACES-1 (NCT00159861) - Sildenafil citrate Co-administered with Epoprostenol
A randomized, double-blind, placebo-controlled study (PACES-1) was conducted in 267 patients with PAH who were taking stable doses of intravenous epoprostenol. Patients had to have a mean pulmonary artery pressure (mPAP) greater than or equal to 25 mmHg and a pulmonary capillary wedge pressure (PCWP) less than or equal to 15 mmHg at rest via right heart catheterization within 21 days before randomization, and a baseline 6-minute walk test distance greater than or equal to 100 meters and less than or equal to 450 meters (mean 349 meters). Patients were randomized to placebo or sildenafil citrate (in a fixed titration starting from 20 mg to 40 mg and then 80 mg, three times a day) and all patients continued intravenous epoprostenol therapy.
At baseline patients had PPH (80%) or PAH secondary to CTD (20%);WHO Functional Class I (1%), II (26%), III (67%), or IV (6%); and the mean age was 48 years, 80% were female, and 79% were Caucasian.
There was a statistically significant greater increase from baseline in 6-minute walk distance at Week 16 (primary endpoint) for the sildenafil citrate group compared with the placebo group. The mean change from baseline at Week 16 (last observation carried forward) was 30 meters for the sildenafil tablet group compared with 4 meters for the placebo group giving an adjusted treatment difference of 26 meters (95% CI: 10.8, 41.2) (p = 0.0009).
Patients on sildenafil citrate achieved a statistically significant reduction in mPAP compared to those on placebo. A mean placebo-corrected treatment effect of -3.9 mmHg was observed in favor of sildenafil tablet (95% CI: -5.7, -2.1) (p = 0.00003).
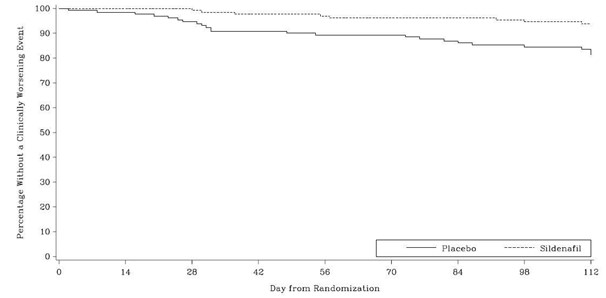
Time to clinical worsening of PAH was defined as the time from randomization to the first occurrence of a clinical worsening event (death, lung transplantation, initiation of bosentan therapy, or clinical deterioration requiring a change in epoprostenol therapy). Table 4 displays the number of patients with clinical worsening events in PACES-1. Kaplan-Meier estimates and a stratified log-rank test demonstrated that placebo-treated patients were 3 times more likely to experience a clinical worsening event than sildenafil citrate -treated patients and that sildenafil citrate -treated patients experienced a significant delay in time to clinical worsening versus placebo-treated patients (p = 0.0074). Kaplan- Meier plot of time to clinical worsening is presented in Figure 5.
Table 3. Clinical Worsening Events in PACES-1 Placebo
(N = 131)
Sildenafil citrate
(N = 134)
Number of patients with clinical
worsening first event23
8
First Event
All Events
First Event
All Events
Death, n
3
4
0
0
Lung transplantation, n
1
1
0
0
Hospitalization due to PAH, n
9
11
8
8
Clinical deterioration resulting in:
Change of Epoprostenol Dose, n
Initiation of Bosentan, n
9
1
16
1
0
0
2
0Proportion worsened
95% Confidence Interval0.187
(0.12 to 0.26)0.062
(0.02 to 0.10)Figure 5. Kaplan-Meier Plot of Time (in Days) to Clinical Worsening of PAH in PACES-1
Improvements in WHO Functional Class for PAH were also demonstrated in patients on sildenafil tablet compared to placebo. More than twice as many sildenafil citrate-treated patients (36%) as placebo-treated patients (14%) showed an improvement in at least one functional New York Heart Association (NYHA) class for PAH.
Study A1481243 (NCT00323297) – Sildenafil citrate Added to Bosentan Therapy – Lack of Effect on Exercise Capacity
A randomized, double-blind, placebo-controlled study was conducted in 103 patients with PAH who were on bosentan therapy for a minimum of 3 months. The PAH patients included those with primary PAH and PAH associated with CTD. Patients were randomized to placebo or sildenafil (20 mg three times a day) in combination with bosentan (62.5 to 125 mg twice a day). The primary efficacy endpoint was the change from baseline at Week 12 in 6-minute walk distance (6MWD). The results indicate that there is no significant difference in mean change from baseline on 6MWD observed between sildenafil 20 mg plus bosentan and bosentan alone.
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Sildenafil 20 mg tablets USP are white to off-white, round biconvex film coated tablets debossed with '85' on one side and plain on other side.
Cartons of 30 tablets (10 tablets each blister pack x 3), NDC 0904-6671-04
Cartons of 50 tablets (10 tablets each blister pack x 5), NDC 0904-6671-06
Recommended Storage for Sildenafil Tablets USP: Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
• Inform patients of contraindication of sildenafil tablets with regular and/or intermittent use of organic nitrates.
• Inform patients that sildenafil is also marketed as VIAGRA for erectile dysfunction. Advise patients taking sildenafil tablets not to take VIAGRA or other PDE-5 inhibitors.
• Advise patients to seek immediate medical attention for a sudden loss of vision in one or both eyes while taking sildenafil tablets. Such an event may be a sign of NAION.
• Advise patients to seek prompt medical attention in the event of sudden decrease or loss of hearing while taking sildenafil tablets. These events may be accompanied by tinnitus and dizziness.
Trademarks are the property of their respective owners.
Dispense with Patient Information available at: https://torrentpharma.com/pi/usa/products/
Manufactured by:
Torrent Pharmaceuticals LTD., India.
Manufactured for:
Torrent Pharma INC., Basking Ridge, NJ 07920.
Packaged and Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268 USA
Refer to package label for Distributor's NDC Number
8098157 Revised: November 2024
-
PATIENT PACKAGE INSERT
Dispense with Patient Information available at: https://torrentpharma.com/pi/usa/products/
This Patient Information has been approved by the U.S. Food and Drug Administration.
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
SILDENAFIL CITRATE
sildenafil citrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0904-6671(NDC:13668-185) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILDENAFIL CITRATE (UNII: BW9B0ZE037) (SILDENAFIL - UNII:3M7OB98Y7H) SILDENAFIL 20 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE (White to off white) Score no score Shape ROUND (Round) Size 7mm Flavor Imprint Code 85 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6671-04 30 in 1 CARTON 11/06/2012 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:0904-6671-06 50 in 1 CARTON 11/06/2012 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091479 11/06/2012 Labeler - Major Pharmaceuticals (191427277)