Label: PIPERACILLIN AND TAZOBACTAM- piperacillin sodium and tazobactam sodium injection, powder, lyophilized, for solution
- NDC Code(s): 55150-119-09, 55150-120-09, 55150-121-09
- Packager: Eugia US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PIPERACILLIN AND TAZOBACTAM FOR INJECTION safely and effectively. See full prescribing information for PIPERACILLIN AND TAZOBACTAM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Intra-abdominal Infections - Piperacillin and tazobactam for injection is indicated in adults and pediatric patients (2 months of age and older) for the treatment of appendicitis (complicated ...
-
2 DOSAGE AND ADMINISTRATION2.2 Dosage in Adult Patients with Indications Other Than Nosocomial Pneumonia - The usual total daily dosage of piperacillin and tazobactam for injection for adult patients with indications other ...
-
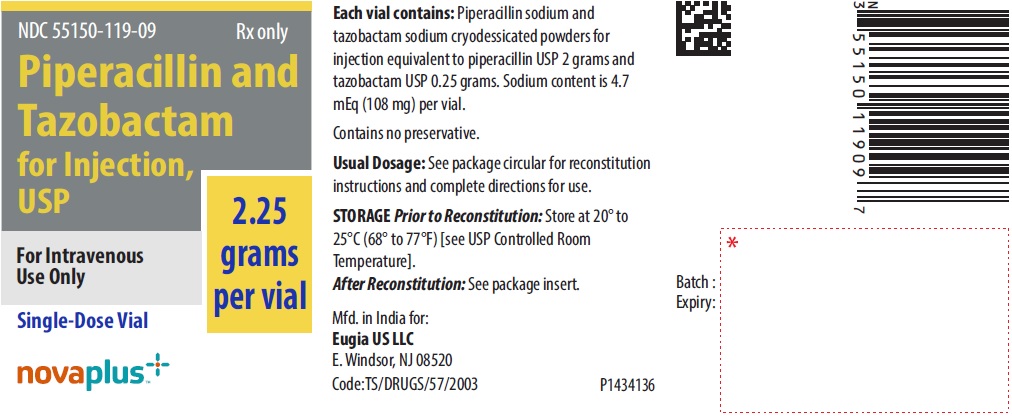
3 DOSAGE FORMS AND STRENGTHSPiperacillin and Tazobactam for Injection, USP is supplied as a white to off-white sterile, cryodesiccated powder in vials: 2.25 g single-dose vial (piperacillin sodium equivalent to 2 grams of ...
-
4 CONTRAINDICATIONSPiperacillin and tazobactam for injection is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or beta-lactamase inhibitors.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Adverse Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Aminoglycosides - Piperacillin may inactivate aminoglycosides by converting them to microbiologically inert amides. In vivo inactivation: When aminoglycosides are administered in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Piperacillin and tazobactam cross the placenta in humans. However, there are insufficient data with piperacillin and/or tazobactam in pregnant women to inform a ...
-
10 OVERDOSAGEThere have been postmarketing reports of overdose with piperacillin/tazobactam. The majority of those events experienced, including nausea, vomiting, and diarrhea, have also been reported with the ...
-
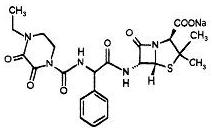
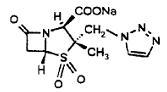
11 DESCRIPTIONPiperacillin and Tazobactam for Injection, USP is an injectable antibacterial combination product consisting of the semisynthetic antibacterial piperacillin sodium and the beta-lactamase inhibitor ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Piperacillin and tazobactam is an antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - The pharmacodynamic parameter for piperacillin/tazobactam that ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term carcinogenicity studies in animals have not been conducted with piperacillin/tazobactam, piperacillin, or ...
-
15 REFERENCES1. Jensen J-US, Hein L, Lundgren B, et al. BMJ Open 2012; 2:e000635. doi:10.1136.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPiperacillin and Tazobactam for Injection, USP is supplied as single-dose vials in the following sizes: Each piperacillin and tazobactam for injection, USP 2.25 g vial provides piperacillin ...
-
17 PATIENT COUNSELING INFORMATIONSerious Hypersensitivity Reactions - Advise patients, their families, or caregivers that serious hypersensitivity reactions, including serious allergic cutaneous reactions, could occur with use ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2.25 grams per vial - Container LabelNDC 55150-119-09 Rx only - Piperacillin and - Tazobactam - for Injection, USP - 2.25 grams per vial - For Intravenous - Use Only - Single-Dose Vial - novaplus+
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2.25 grams sper vial - Container-Carton (10 Vials)NDC 55150-119-09 - Piperacillin and Tazobactam - for Injection, USP - 2.25 grams - per vial - For Intravenous Use Only - 10 x 2.25 gram Single-Dose Vials - Rx only - novaplus+
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3.375 grams per vial - Container LabelNDC 55150-120-09 Rx only - Piperacillin and - Tazobactam - for Injection, USP - 3.375 grams per vial - For Intravenous Use Only - Single-Dose Vial - novaplus+
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3.375 grams per vial - Container-Carton (10 Vials)NDC 55150-120-09 - Piperacillin and Tazobactam - for Injection, USP - 3.375 grams per vial - For Intravenous Use Only - 10 x 3.375 gram Single-Dose Vials - Rx only novaplus+
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4.5 grams per vial - Container LabelNDC 55150-121-09 Rx only - Piperacillin and - Tazobactam - for Injection, USP - 4.5 grams per vial - For Intravenous - Use Only - Single-Dose Vial - novaplus+
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4.5 grams per vial - Container-Carton (10 Vials)NDC 55150-121-09 - Piperacillin and Tazobactam - for Injection, USP - 4.5 grams per vial - For Intravenous Use Only - 10 x 4.5 gram Singl-Dose Vials - Rx only - novaplus+
-
INGREDIENTS AND APPEARANCEProduct Information