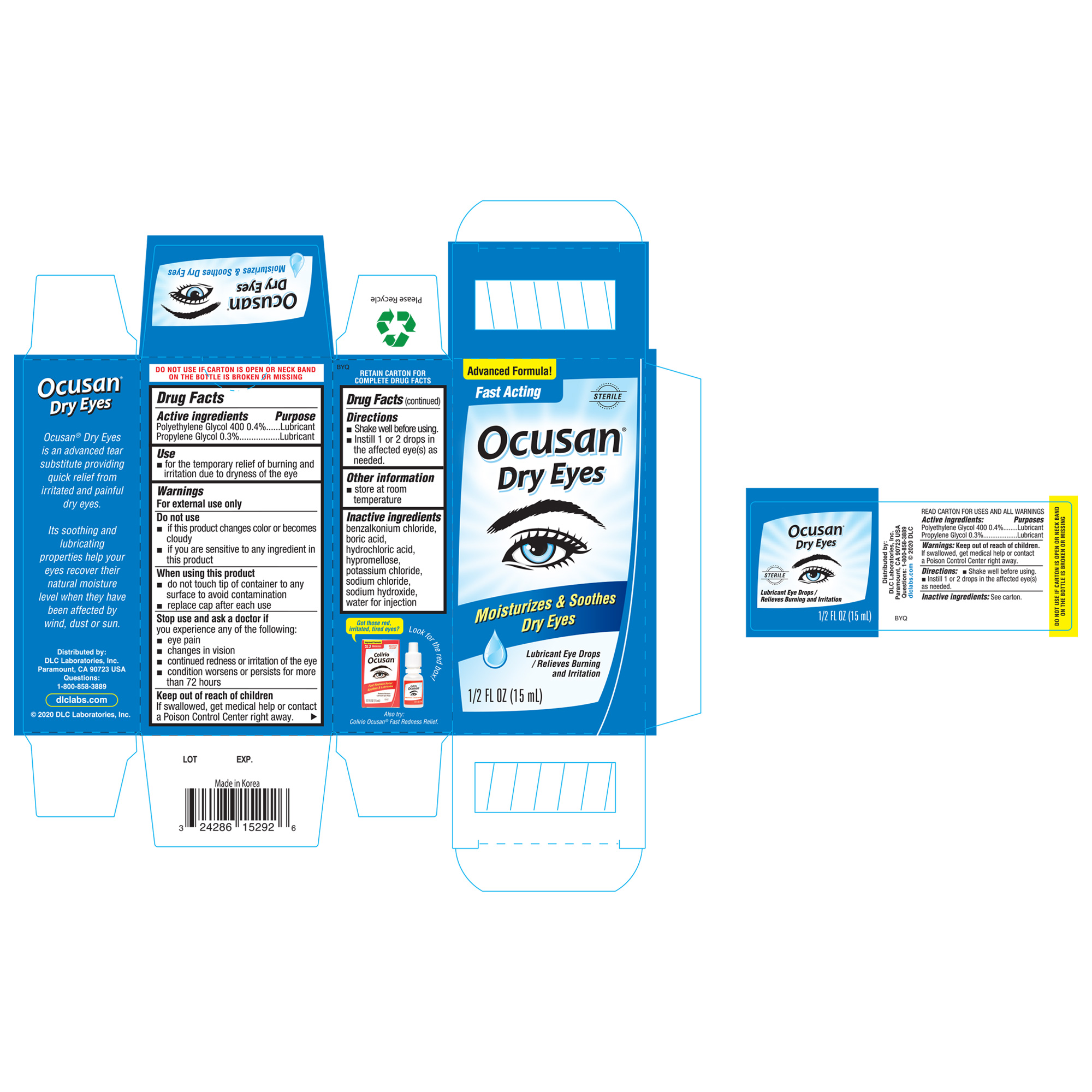
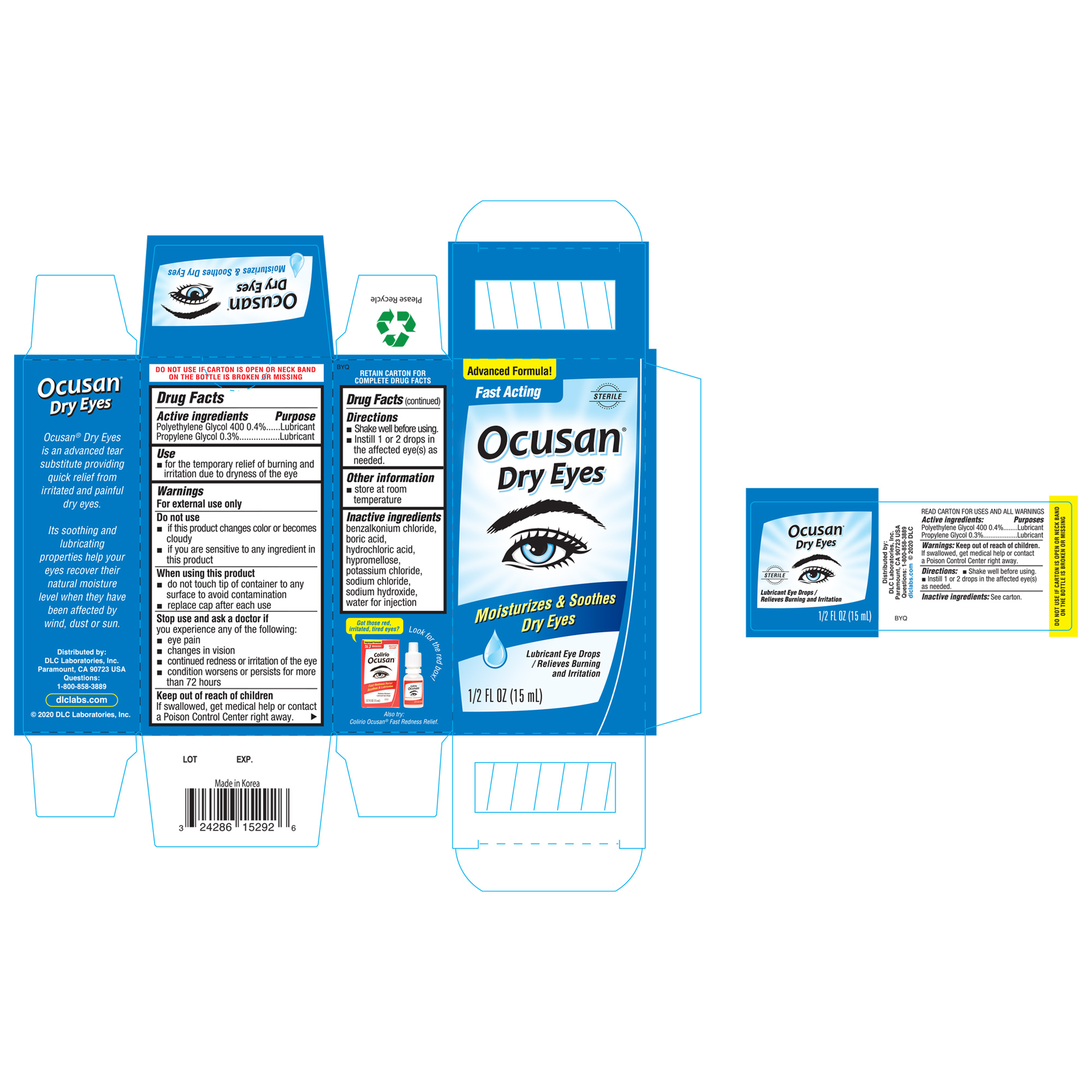
Label: OCUSAN DRY EYES- polyethylene glycol 400, propylene glycol liquid
- NDC Code(s): 24286-1292-5
- Packager: DLC Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 18, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
ACTIVE INGREDIENTActive ingredientsPurposes - Polyethylene Glycol 400 1%Lubricant - proplyene GlycolLubricant
-
Usefor the temporary relief of burning and irritation due to dryness of the eye
-
WarningsFor external use only - Do Not Use - if this product changes color or becomes cloudy - if you are sensitive to any ingredients in this product - When using this product - do not touch tip of ...
-
Directionsshake well before using - instill 1 to 2 drops in the affected eye(s) as needed
-
Other informationstore at room temperature
-
Inactive ingredientsbenzalkonium chloride, boric acid, hydrochloric acid, hypromellose, potassium chloride, sodium chloride, sodium hydroxide, water for injection
-
Questions1-800-858-3889
-
SPL UNCLASSIFIED SECTIONDistributed by: DLC LABORATORIES, INC. PARAMOUNT, CA 90723 USA
-
PRINCIPAL DISPLAY PANEL - 15 mL Bottle BoxAdvanced Formula! Fase Acting - Ocusan - ® Dry Eyes - Moisturizes & Soothes Dry Eyes - Lubricant Eye Drops / Relieves Buring and Irritation - STERILE - 1/2 FL OZ (15 mL)
-
INGREDIENTS AND APPEARANCEProduct Information