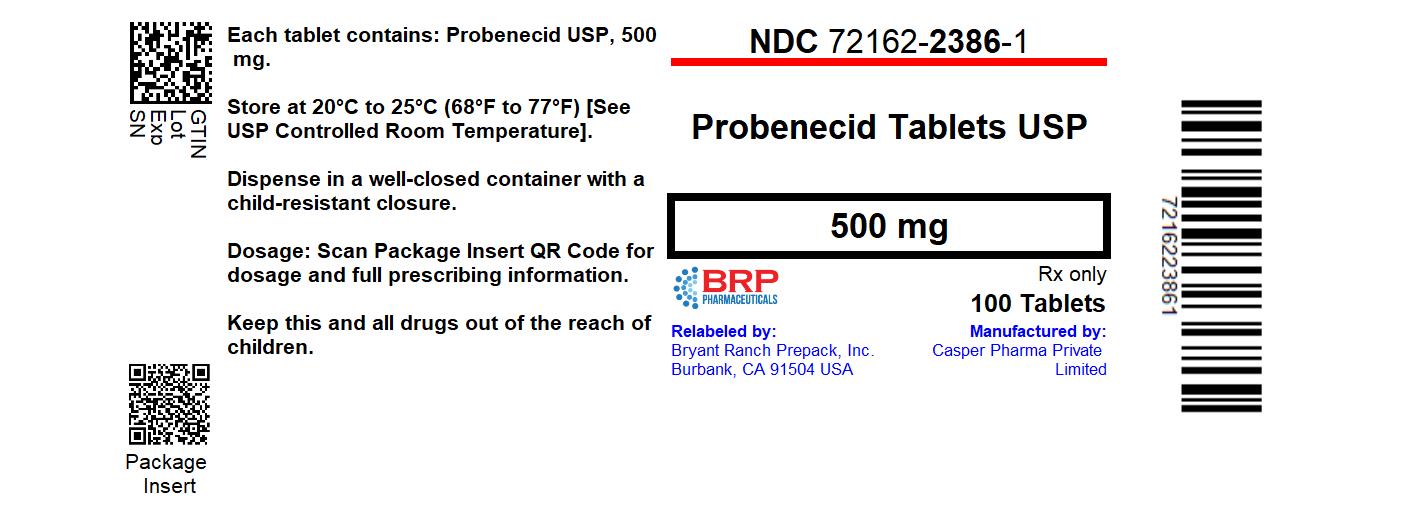
Label: PROBENECID tablet
- NDC Code(s): 72162-2386-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 16571-831
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
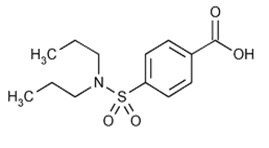
DESCRIPTIONProbenecid is a uricosuric and renal tubular transport blocking agent. Probenecid is the generic name for 4-[(dipropyl-amino)sulfonyl] benzoic acid. It has the following structural ...
-
CLINICAL PHARMACOLOGYProbenecid is a uricosuric and renal tubular blocking agent. It inhibits the tubular reabsorption of urate, thus increasing the urinary excretion of uric acid and decreasing serum urate levels ...
-
INDICATIONS & USAGEProbenecid tablets are indicated for the treatment of the hyperuricemia associated with gout and gouty arthritis. As an adjuvant to therapy with penicillin or with ampicillin, methicillin ...
-
CONTRAINDICATIONSHypersensitivity to probenecid. Children under 2 years of age. Not recommended in persons with known blood dyscrasias or uric acid kidney stones. Therapy with probenecid should not be ...
-
WARNINGSExacerbation of gout following therapy with probenecid may occur; in such cases colchicine or other appropriate therapy is advisable. Probenecid increases plasma concentrations of methotrexate ...
-
PRECAUTIONSGENERAL PRECAUTIONS - Hematuria, renal colic, costovertebral pain, and formation of uric acid stones associated with the use of probenecid in gouty patients may be prevented by alkalization of ...
-
ADVERSE REACTIONSThe following adverse reactions have been observed and within each category are listed in order of decreasing severity. Central Nervous System: headache, dizziness. Metabolic: precipitation of ...
-
DOSAGE & ADMINISTRATIONGout - Therapy with probenecid should not be started until an acute gouty attack has subsided. However, if an acute attack is precipitated during therapy, probenecid may be continued without ...
-
HOW SUPPLIEDProbenecid Tablets USP, 500 mg are bisected, capsule-shaped, yellow, film-coated tablets debossed with “C84” on one side and break line on other side. NDC: 72162-2386-1: 100 Tablets in a ...
-
PRINCIPAL DISPLAY PANELProbenecid 500 Tablets #100
-
INGREDIENTS AND APPEARANCEProduct Information