Label: ACYCLOVIR suspension
- NDC Code(s): 0472-0082-16
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
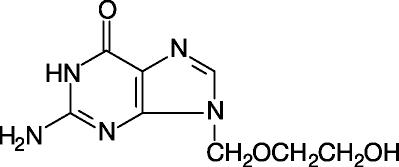
Acyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir suspension is formulated for oral administration. Each teaspoonful (5 mL) of acyclovir oral suspension, USP ...
-
CLINICAL PHARMACOLOGY
Pharmacokinetics: The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster ...
-
INDICATIONS AND USAGE
Herpes Zoster Infections: Acyclovir is indicated for the acute treatment of herpes zoster (shingles). Genital Herpes: Acyclovir is indicated for the treatment of initial episodes and the ...
-
CONTRAINDICATIONS
Acyclovir oral suspension is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
-
WARNINGS
Acyclovir oral suspension is intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see ADVERSE REACTIONS: Observed During ...
-
PRECAUTIONS
Dosage adjustment is recommended when administering acyclovir to patients with renal impairment (see DOSAGE AND ADMINISTRATION). Caution should also be exercised when administering acyclovir to ...
-
ADVERSE REACTIONS
Herpes Simplex: Short-Term Administration: The most frequent adverse events reported during clinical trials of treatment of genital herpes with acyclovir 200 mg administered orally 5 times daily ...
-
OVERDOSAGE
Overdoses involving ingestion of up to 100 capsules (20 g) have been reported. Adverse events that have been reported in association with overdosage include agitation, coma, seizures, and ...
-
DOSAGE AND ADMINISTRATION
Acute Treatment of Herpes Zoster: 800 mg every 4 hours orally, 5 times daily for 7 to 10 days. Genital Herpes: Treatment of Initial Genital Herpes: 200 mg every 4 hours, 5 times daily for 10 ...
-
HOW SUPPLIED
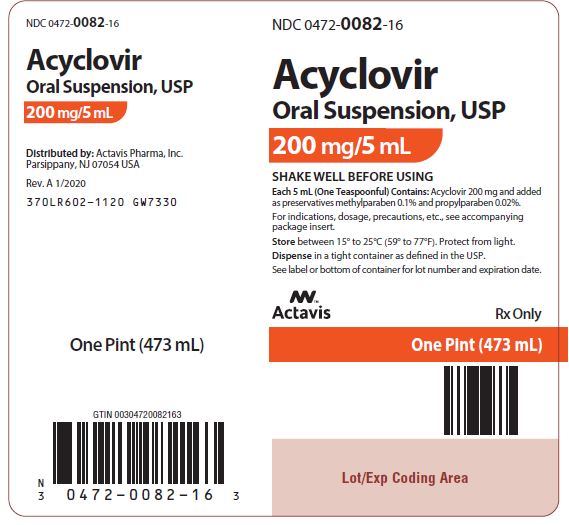
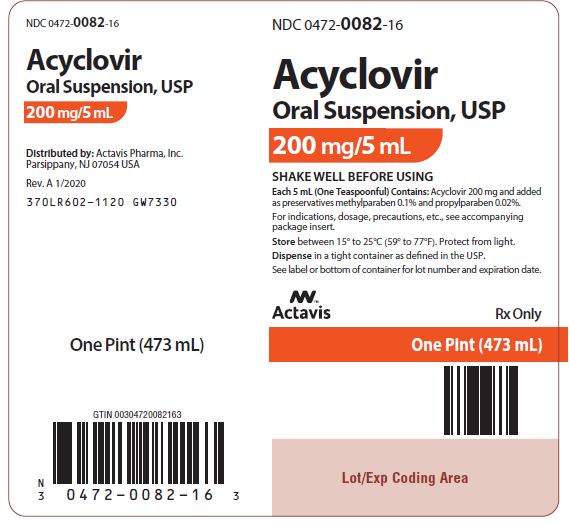
Acyclovir oral suspension, USP (off-white, banana-flavored) containing 200 mg acyclovir in each teaspoonful (5 mL). Bottle of 1 pint (473 mL) NDC 0472-0082-16. Store between 15° to 25°C (59° to ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0472-0082-16 - Acyclovir Oral Suspension, USP - 200 mg/5 mL - SHAKE WELL BEFORE USING - Each 5 mL (One Teaspoonful) Contains: Acyclovir 200 mg and added as preservatives methylparaben 0.1% and ...
-
INGREDIENTS AND APPEARANCEProduct Information