Label: TUBERSOL- tuberculin purified protein derivative injection, solution
- NDC Code(s): 49281-752-21, 49281-752-22, 49281-752-78, 49281-752-98
- Packager: Sanofi Pasteur Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONAHFS Category: 36:84 - Rx only - Diagnostic Antigen - (Aid in the detection of infection with Mycobacterium tuberculosis) FOR INTRADERMAL USE - Polysorbate 80 Stabilized Solution of Tuberculin ...
-
DESCRIPTIONTUBERSOL® Tuberculin Purified Protein Derivative (Mantoux) (PPD) (1) for intradermal tuberculin testing is prepared from a large Master Batch Connaught Tuberculin (CT68) (2) and is a cell-free ...
-
CLINICAL PHARMACOLOGYMechanism of Action - The sensitization following infection with mycobacteria occurs primarily in the regional lymph nodes. Small lymphocytes (T lymphocytes) proliferate in response to the ...
-
INDICATIONS AND USAGETUBERSOL Tuberculin Purified Protein Derivative (Mantoux), is indicated to aid diagnosis of tuberculosis infection (TB) in persons at increased risk of developing active disease. The Centers for ...
-
CONTRAINDICATIONSAllergy to any component of TUBERSOL or an anaphylactic or other allergic reaction to a previous test of tuberculin PPD is a contraindication to the use of TUBERSOL. (See DESCRIPTION and HOW ...
-
WARNINGSHypersensitivity - Allergic reactions may occur following the use of TUBERSOL even in persons with no prior history of hypersensitivity to the product components. (10) Epinephrine injection ...
-
PRECAUTIONSGeneral - Diagnostic Limitations - False positive or false negative tuberculin skin test reactions may occur in some individuals. (See Interpretation of the Test.) False positive tuberculin ...
-
ADVERSE REACTIONSInduration at the TUBERSOL injection site is the expected reaction for a positive skin test. (See Interpretation of the Test.) The information pertaining to adverse events has been compiled from ...
-
DOSAGE AND ADMINISTRATIONDosage - Five (5) tuberculin units (TU) per test dose of 0.1 mL is the standard strength used for intradermal (Mantoux) testing. Method of Administration - TUBERSOL is indicated for ...
-
HOW SUPPLIEDTUBERSOL Tuberculin Purified Protein Derivative (Mantoux), bioequivalent to 5 US units (TU) PPD-S per test dose (0.1 mL) is supplied in: 1 mL multi-dose vial (10 tests). NDC No. 49281-752-78 ...
-
REFERENCES1 - Landi S. Preparation, purification, and stability of tuberculin. Appl Microbiol 1963;11:408-412. 2 - Landi S, et al. Preparation and characterization of a large batch of tuberculin purified ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Sanofi Pasteur Limited - Toronto Ontario Canada - Distributed by: Sanofi Pasteur Inc. Swiftwater PA 18370 USA - Product Information as of - October 2021 - TUBERSOL is a registered ...
-
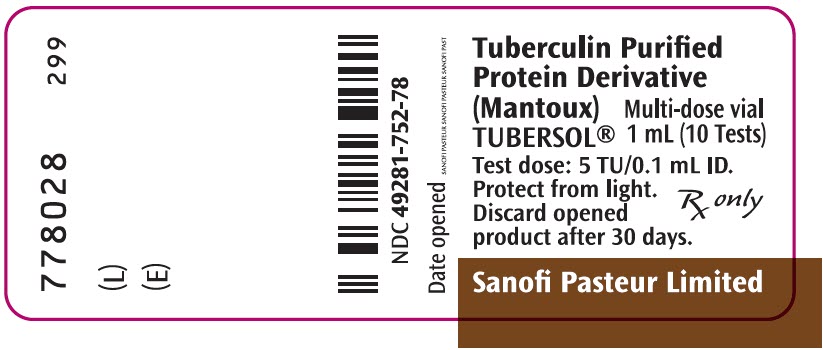
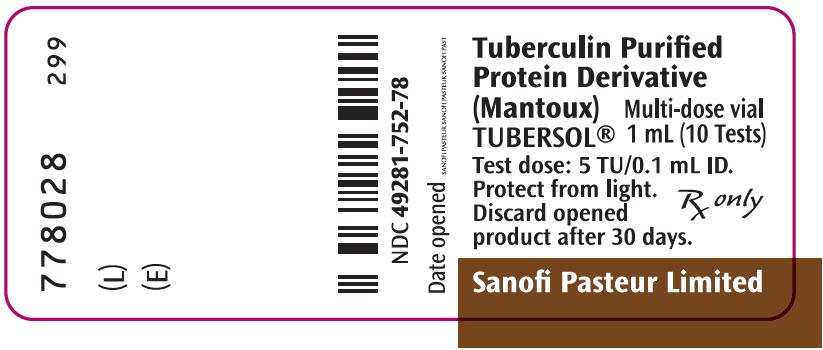
PRINCIPAL DISPLAY PANEL - 1 mL Vial LabelTuberculin Purified - Protein Derivative - (Mantoux) TUBERSOL® Multi-dose vial - 1 mL (10 Tests) Test dose: 5 TU/0.1 mL ID. Protect from light. Discard opened - product after 30 days. Rx only - Sanofi ...
-
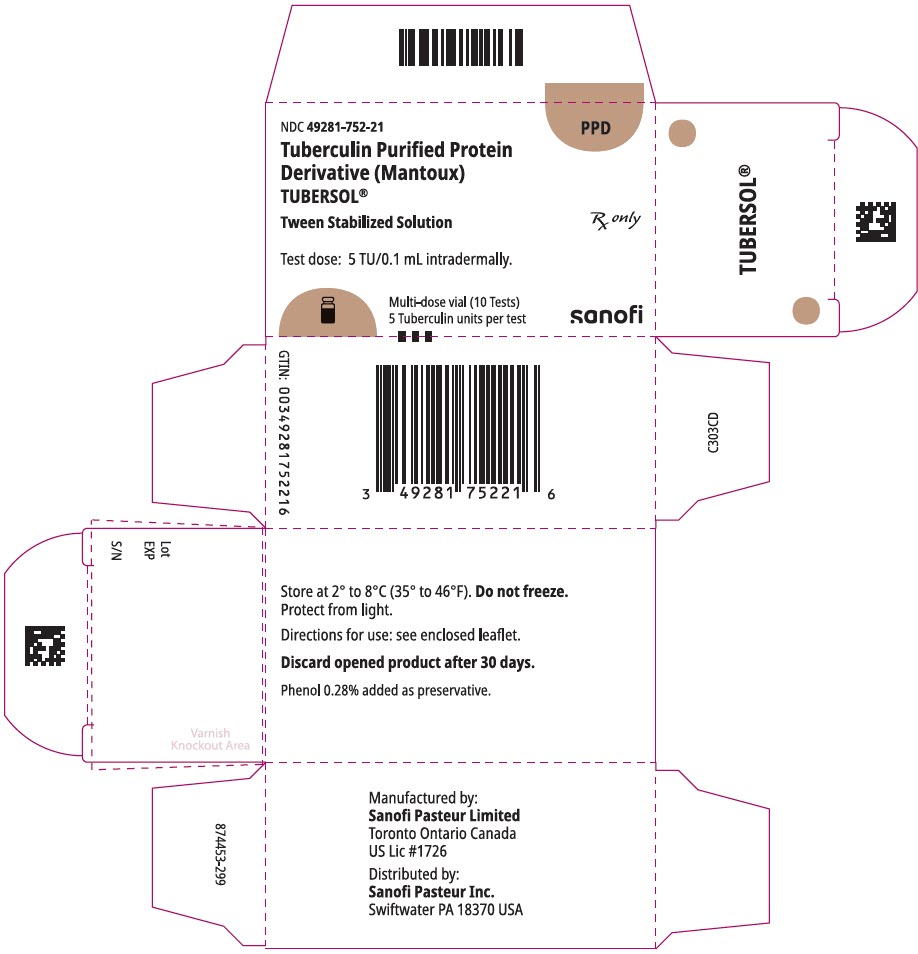
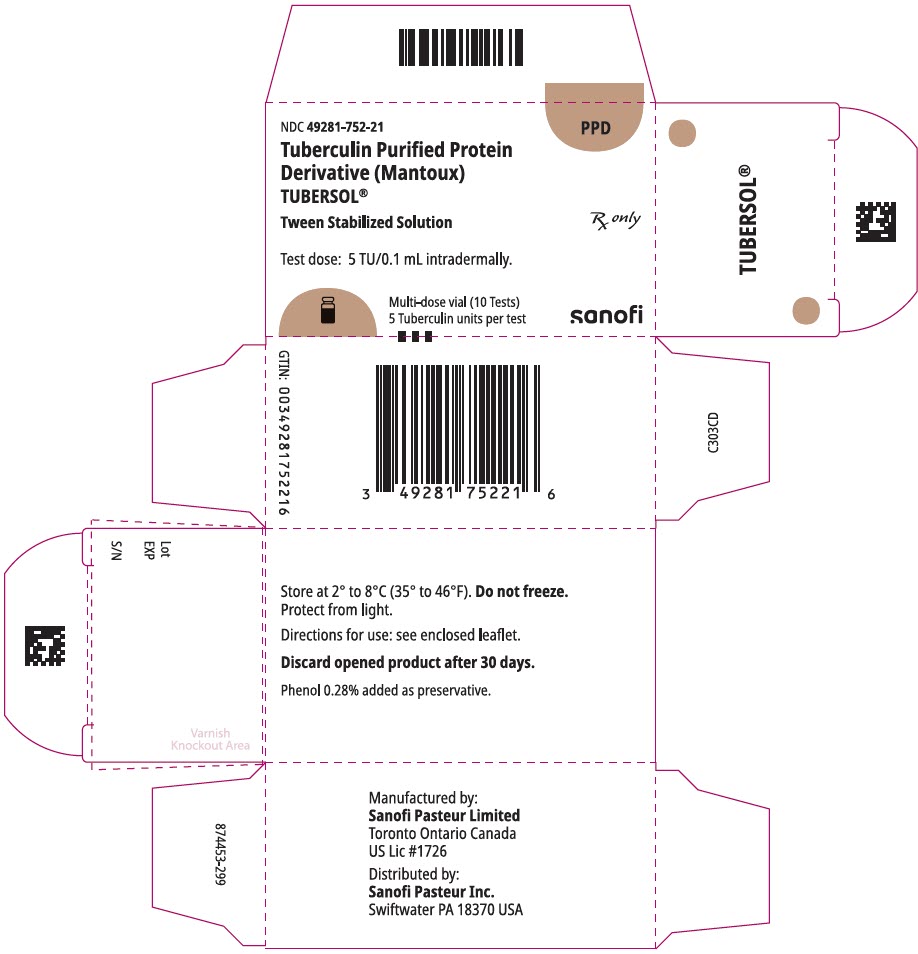
PRINCIPAL DISPLAY PANEL - 1 mL Vial CartonNDC 49281-752-21 - PPD - Tuberculin Purified Protein - Derivative (Mantoux) TUBERSOL® Tween Stabilized Solution - Test dose: 5 TU/0.1 mL intradermally. Multi-dose vial (10 Tests) 5 Tuberculin units per ...
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial LabelTuberculin Purified Protein - Derivative (Mantoux) TUBERSOL® Multi-dose vial - 5 mL (50 Tests) Test dose: 5 TU/0.1 mL ID. Protect from light. Discard opened product after 30 days. Rx only - Sanofi ...
-
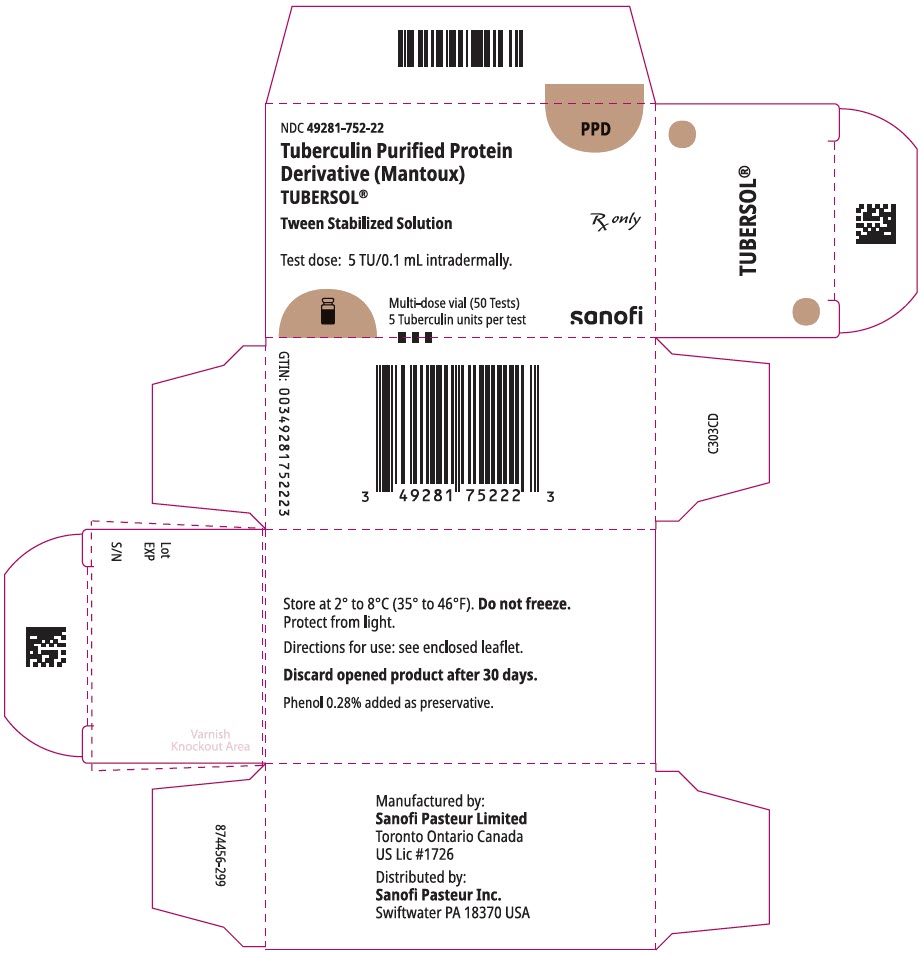
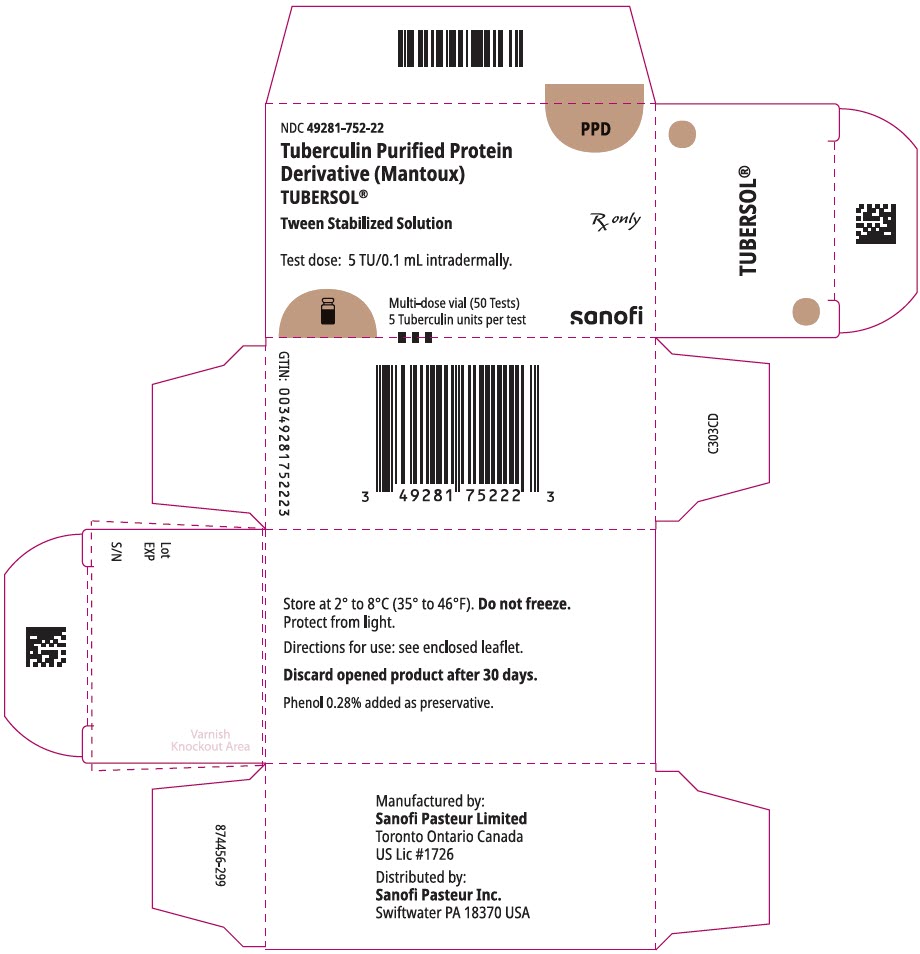
PRINCIPAL DISPLAY PANEL - 5 mL Vial CartonNDC 49281-752-22 - PPD - Tuberculin Purified Protein - Derivative (Mantoux) TUBERSOL® Tween Stabilized Solution - Rx only - Test dose: 5 TU/0.1 mL intradermally. Multi-dose vial (50 Tests) 5 Tuberculin ...
-
INGREDIENTS AND APPEARANCEProduct Information