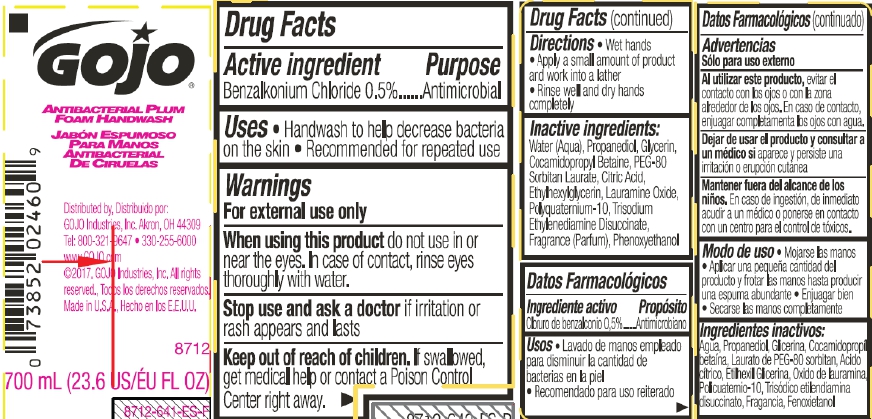
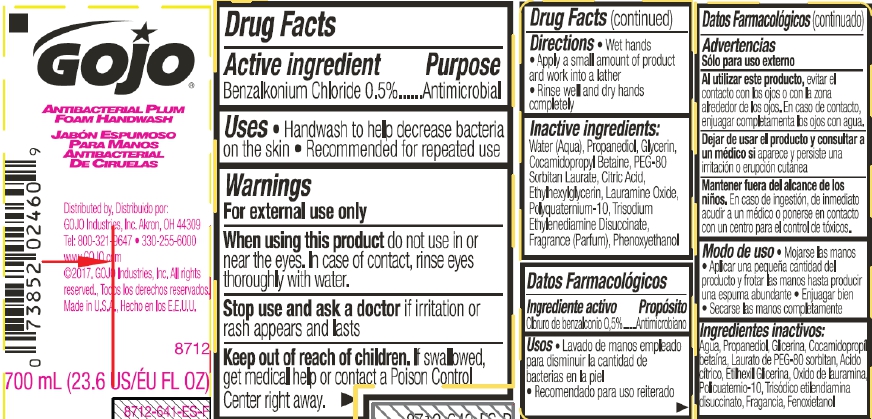
Label: GOJO ANTIBACTERIAL PLUM FOAM HANDWASH- benzalkonium chloride liquid
- NDC Code(s): 21749-968-08, 21749-968-89, 21749-968-90, 21749-968-97
- Packager: GOJO Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOJO ANTIBACTERIAL PLUM FOAM HANDWASH
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21749-968 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.5 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PEG-80 SORBITAN LAURATE (UNII: 239B50Y732) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) POLYQUATERNIUM-10 (10000 MPA.S AT 2%) (UNII: PI1STR9QYH) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-968-08 236 mL in 1 PACKAGE; Type 0: Not a Combination Product 06/14/2017 04/30/2018 2 NDC:21749-968-97 700 mL in 1 PACKAGE; Type 0: Not a Combination Product 06/14/2017 3 NDC:21749-968-89 1200 mL in 1 PACKAGE; Type 0: Not a Combination Product 06/14/2017 4 NDC:21749-968-90 1250 mL in 1 PACKAGE; Type 0: Not a Combination Product 06/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/14/2017 Labeler - GOJO Industries, Inc. (004162038) Establishment Name Address ID/FEI Business Operations GOJO Industries, Inc. 036424534 manufacture(21749-968) Establishment Name Address ID/FEI Business Operations GOJO Industries, Inc. 088312414 manufacture(21749-968) , label(21749-968) , pack(21749-968) Establishment Name Address ID/FEI Business Operations Travis Association for the Blind 026032268 label(21749-968)